N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Characterization of Catalysts
2.3. Photocatalytic Activity
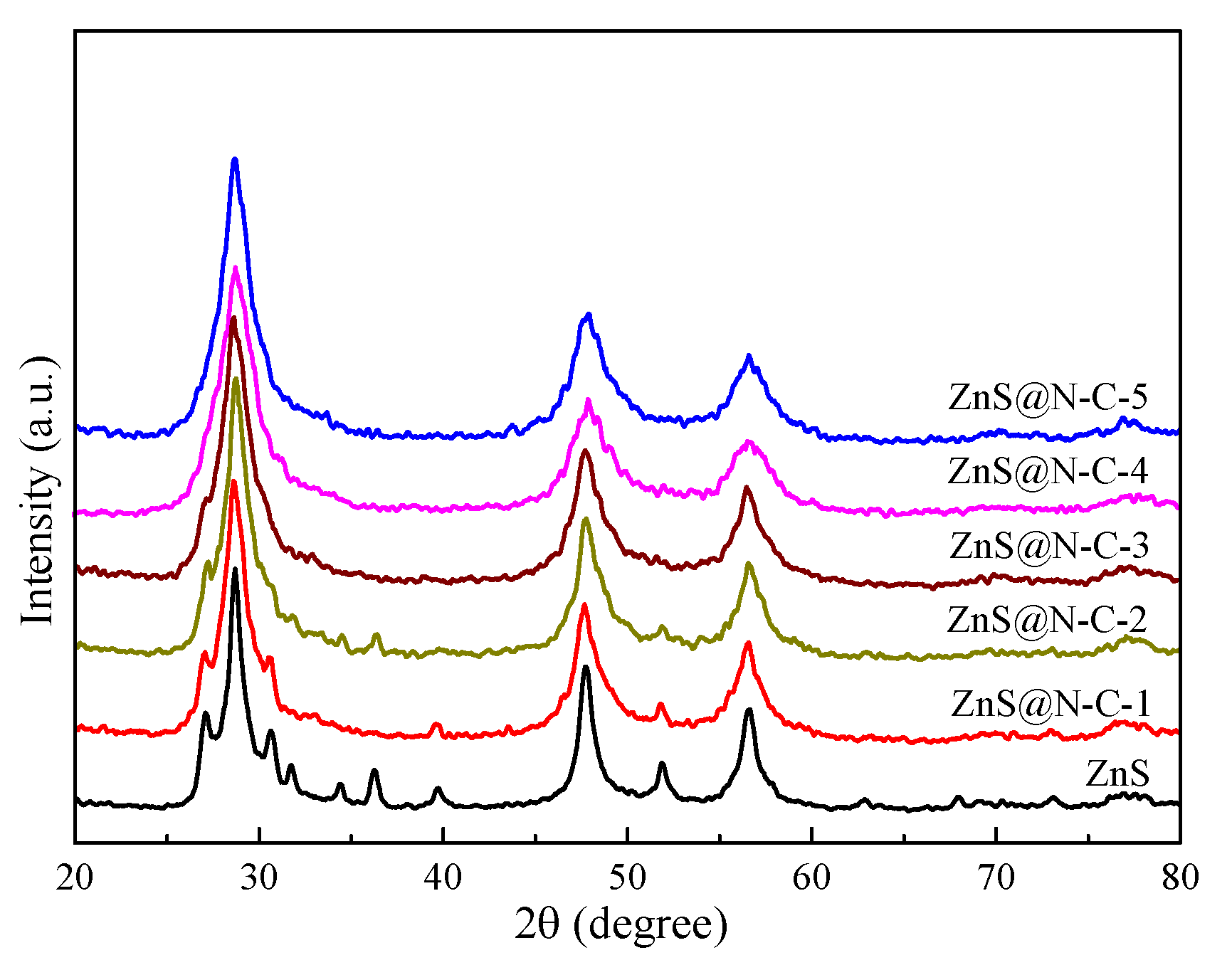
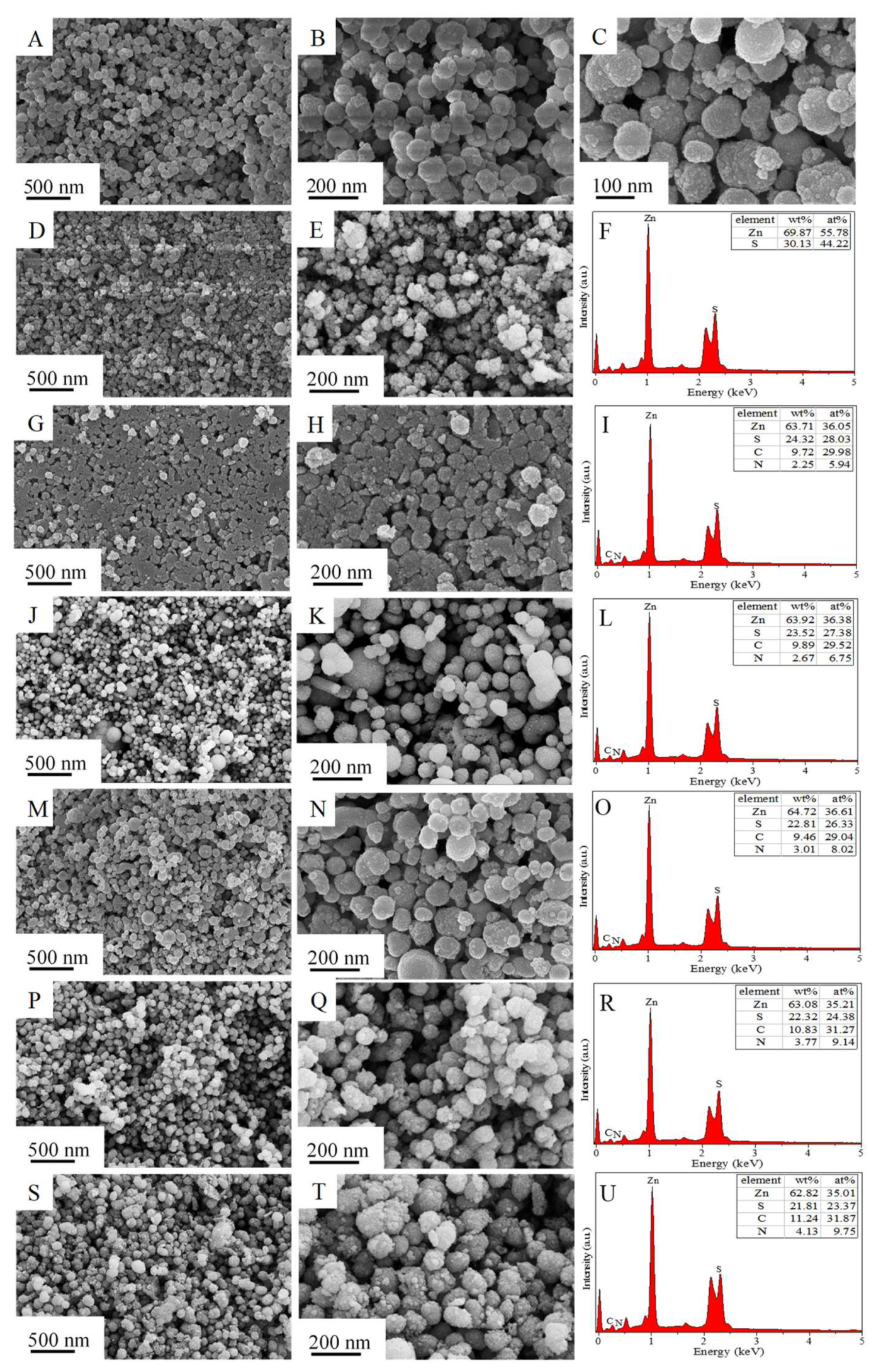
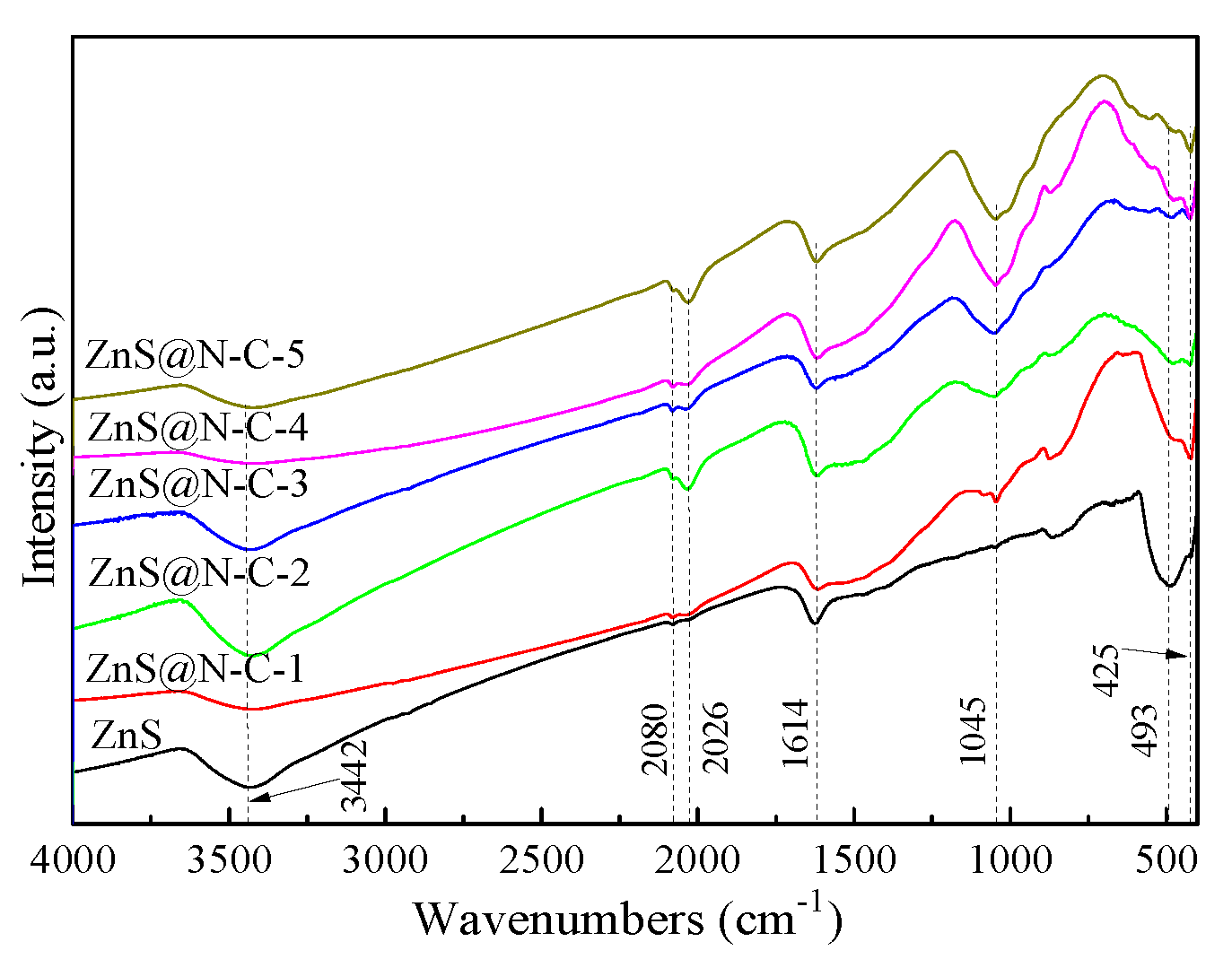
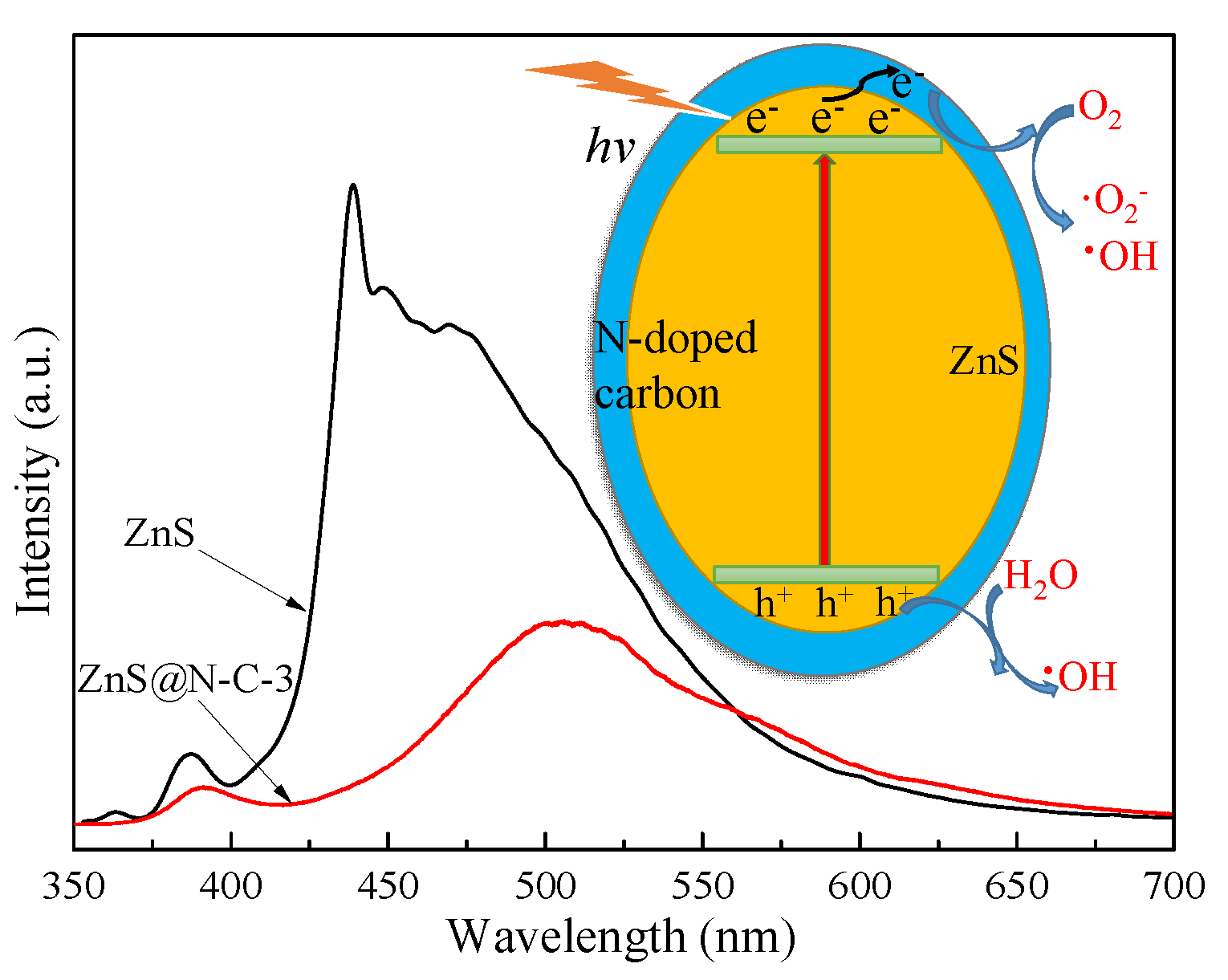
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Y.; Lv, C.; Hou, J.; Yuan, S.; Wang, Y.; Xu, P.; Gao, G.; Shi, J. 3D hollow hierarchical structures based on 1D BiOCl nanorods intersected with 2D Bi2WO6 nanosheets for efficient photocatalysis under visible light. Nanomaterials 2019, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Durán, A.; Martín, I.S.; Carrillo, P. Effect of sodium persulfate as electron acceptor on antipyrine degradation by solar TiO2 or TiO2/rGO photocatalysis. Chem. Eng. J. 2019, 364, 257–268. [Google Scholar] [CrossRef]
- Smykalova, A.; Sokolova, B.; Foniok, K.; Matejka, V.; Praus, P. Photocatalytic degradation of selected pharmaceuticals using g-C3N4 and TiO2 nanomaterials. Nanomaterials 2019, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fu, W.; Kang, F.; Peng, H.; Wen, J. Enhanced photo-Fenton degradation of tetracycline using TiO2-coated α-Fe2O3 core–shell heterojunction. J. Ind. Eng. Chem. 2018, 68, 14–23. [Google Scholar] [CrossRef]
- Wei, B.; Tielens, F.; Calatayud, M. Understanding the role of rutile TiO2 surface orientation on molecular hydrogen activation. Nanomaterials 2019, 9, 1199. [Google Scholar] [CrossRef]
- Boumaza, S.; Kabir, H.; Gharbi, I.; Belhadi, A.; Trari, M. Preparation and photocatalytic H2-production on α-Fe2O3 prepared by sol-gel. Int. J. Hydrog. Energy 2018, 43, 3424–3430. [Google Scholar] [CrossRef]
- Touati, A.; Hammedi, T.; Najjar, W.; Ksibi, Z.; Sayadi, S. Photocatalytic degradation of textile wastewater in presence of hydrogen peroxide: Effect of cerium doping titania. J. Ind. Eng. Chem. 2016, 35, 36–44. [Google Scholar] [CrossRef]
- Lyu, J.; Hu, Z.; Li, Z.; Ge, M. Removal of tetracycline by BiOBr microspheres with oxygen vacancies: Combination of adsorption and photocatalysis. J. Phys. Chem. Solids 2019, 129, 61–70. [Google Scholar] [CrossRef]
- Tang, J.; Yang, D.; Zhou, W.; Guo, R.; Pan, W.; Huang, C. Noble-metal-free molybdenum phosphide co-catalyst loaded graphitic carbon nitride for efficient photocatalysis under simulated irradiation. J. Catal. 2019, 370, 79–87. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, X.; Luo, Y.; Xu, P.; He, J.; Jiang, L.; Li, J.; Yan, Z.; Wang, J. Efficient charge carrier separation in l-alanine acids derived N-TiO2 nanospheres: The role of oxygen vacancies in tetrahedral Ti4+ sites. Nanomaterials 2019, 9, 698. [Google Scholar] [CrossRef]
- Yu, L.; Ba, X.; Qiu, M.; Li, Y.; Shuai, L.; Zhang, W.; Ren, Z.; Yu, Y. Visible-light driven CO2 reduction coupled with water oxidation on Cl-doped Cu2O nanorods. Nano Energy 2019, 60, 576–582. [Google Scholar] [CrossRef]
- Kaur, J.; Gupta, A.; Pandey, O.P. Photocatalytic study of ZnS-Ag2S nanocomposites-effect of thioglycerol. Sol. Energy 2018, 176, 678–687. [Google Scholar] [CrossRef]
- Rameshbabu, R.; Ravi, P.; Sathish, M. Cauliflower-like CuS/ZnS nanocomposites decorated g-C3N4 nanosheets as noble metal-free photocatalyst for superior photocatalytic water splitting. Chem. Eng. J. 2018, 176, 678–687. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Hu, X.; Deng, F.; Huang, W.; Zeng, G.; Luo, X.; Dionysiou, D.D. The band structure control of visible-light-driven rGO/ZnS-MoS2 for excellent photocatalytic degradation performance and long-term stability. Chem. Eng. J. 2018, 350, 248–256. [Google Scholar] [CrossRef]
- Li, P.; He, T. Common-cation based Z-scheme ZnS@ZnO core-shell nanostructure for efficient solar-fuel production. Appl. Catal. B Environ. 2018, 238, 518–524. [Google Scholar] [CrossRef]
- Deka, D.C.; Kalita, A.; Bardaloi, S.; Kalitab, M.P.C. Influence of capping agent on structural, optical and photocatalytic properties of ZnS nanocrystals. J. Lumin. 2019, 210, 269–275. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Zhao, S.; Chen, X.; Yu, Y. Synergistic effect of adsorption and visible-light photocatalysis for organic pollutant removal over BiVO4/carbon sphere nanocomposites. Appl. Surf. Sci. 2018, 453, 394–404. [Google Scholar] [CrossRef]
- Choi, J.; Kim, N.; Oh, J.; Kim, F.S. Bandgap engineering of nanosized carbon dots through electron-accepting functionalization. J. Ind. Eng. Chem. 2018, 65, 104–111. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, K.; Huang, Z.; Liu, Y.; Wen, J.; Peng, H. MgO nanosheets with N-doped carbon coating for the efficient visible-light photocatalysis. J. Ind. Eng. Chem. 2019, 76, 288–295. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Li, J.; Sun, L.; Zhou, Y.; Guan, J.; Wang, H.; Huo, P.; Ma, C.; Yan, Y. Carbon dots modifying sphere-flower CdIn2S4 on N-rGO sheet muti-dimensional photocatalyst for efficient visible degradation of 2,4-dichlorophenol. J. Taiwan Inst. Chem. Eng. 2019, 99, 142–153. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Peng, X.; Wang, Z.; Zhou, L.; Yin, Q. A novel route to manufacture 2D layer MoS2 and g-C3N4 by atmospheric plasma with enhanced visible-light-driven photocatalysis. Nanomaterials 2019, 9, 1139. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Benyounes, A.; Serp, P.; Faria, J.L.; Silva, C.G. Photocatalytic synthesis of vanillin using N-doped carbon nanotubes/ZnO catalysts under UV-LED irradiation. Appl. Catal. A Gen. 2018, 551, 71–78. [Google Scholar] [CrossRef]
- Appavu, B.; Kannan, K.; Thiripuranthagan, S. Enhanced visible light photocatalytic activities of template free mesoporous nitrogen doped reduced graphene oxide/titania composite catalysts. J. Ind. Eng. Chem. 2016, 36, 184–193. [Google Scholar] [CrossRef]
- Hsu, C.; Li, C.; Zhang, L.; Lu, S. N-doped carbon dots@layer facilitated heterostructure of TiO2 polymorphs for efficient photoelectrochemical water oxidation. J. Taiwan Inst. Chem. Eng. 2018, 93, 388–396. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, S.; Li, X.; Kong, C.; Guo, Z.; Chen, L. In situ fabrication of ZnO@N-doped nanoporous carbon core-shell heterostructures with high photocatalytic and adsorption capacity by a calcination of ZnO@MOF strategy. J. Solid State Chem. 2017, 255, 108–114. [Google Scholar] [CrossRef]
- Lin, X.; Liu, B.; Huang, H.; Shi, C.; Liu, Y.; Kang, Z. One-step synthesis of ZnS-N/C nanocomposites derived from Zn-based chiral metal–organic frameworks with highly efficient photocatalytic activity for the selective oxidation cis-cyclooctene. Inorg. Chem. Front. 2018, 5, 723–731. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Alshehri, S.M.; Naushad, M.; Ahamad, R.T. Fabrication of highly porous N/S doped carbon embedded with ZnS as highly efficient photocatalyst for degradation of bisphenol. Int. J. Biol. Macromol. 2019, 121, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Nekouei, S.; Nekouei, F. Comparative procedure of photodegradation of methylene blue using N doped activated carbon loaded with hollow 3D flower like ZnS in two synergic phases of adsorption and catalytic. J. Photoch. Photobio. A 2018, 364, 262–273. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Wang, H.; Jiang, Y.; Jin, L.; Guo, Y.; Song, Y.; Fang, F.; Sun, D. Construction of hybrid hollow architectures by in-situ rooting ultrafine ZnS nanorods within porous carbon polyhedra for enhanced lithium storage properties. Chem. Eng. J. 2017, 326, 680–690. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, Y.; Li, Z.; Dong, Y.; Zhang, J.; Wen, J.; Peng, H. Sm2O3 nanoparticles coated with N-doped carbon for enhanced visible-light photocatalysis. J. Phys. Chem. Solids 2019, 130, 180–188. [Google Scholar] [CrossRef]
- Zheng, X.; Mao, Y.; Wen, J.; Fu, X.; Liu, X. CuInS2/Mg(OH)2 nanosheets for the enhanced visible-light photocatalytic degradation of tetracycline. Nanomaterials 2019, 9, 1567. [Google Scholar] [CrossRef]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloy. Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Zeng, G.; Xue, W.; Chen, S.; Li, Z.; Deng, R.; Yang, Y.; Cheng, M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. Chem. Eng. J. 2019, 375, 121991. [Google Scholar] [CrossRef]
- Jiang, X.; Lai, S.; Xu, W.; Fang, J.; Chen, X.; Beiyuan, J.; Zhou, X.; Lin, K.; Liu, J.; Guan, G. Novel ternary BiOI/g-C3N4/CeO2 catalysts for enhanced photocatalytic degradation of tetracycline under visible-light radiation via double charge transfer process. J. Alloy. Compd. 2019, 809, 151804. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, G.; Yin, C.; Li, C.; Zheng, S. Facile fabrication of heterogeneous TiO2/BiOCl composite with superior visible-light-driven performance towards Cr(VI) and tetracycline. Mater. Res. Bull. 2019, 119, 110559. [Google Scholar] [CrossRef]
- Yu, X.; Yu, J.; Cheng, B.; Huang, B. One-pot template-free synthesis of monodisperse zinc sulfide hollow spheres and their photocatalytic properties. Chem. Eur. J. 2009, 15, 6731–6739. [Google Scholar] [CrossRef]
- Huang, F.; Banfield, J.F. Size-dependent phase transformation kinetics in nanocrystalline ZnS. J. Am. Chem. Soc. 2005, 127, 4523–4529. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, X.; Peng, H.; Wen, J. Ag-decorated core-shell Sm2O3@TiO2 nanocomposites with enhanced visible-light photocatalytic performance. J. Phys. Chem. Solids 2018, 123, 206–215. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Z.; Peng, H.; Zheng, X. Core-shell Sm2O3@ZnO nano-heterostructure for the visible light driven photocatalytic performance. Colloid. Surface. A 2019, 560, 244–251. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; You, Y.; Peng, H.; Wen, J. Core-shell structured α-Fe2O3@CeO2 heterojunction for the enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2018, 101, 20–28. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; You, Y.; Fu, X.; Wen, J.; Zheng, X. NiFe2O4/g-C3N4 heterojunction composite with enhanced visible-light photocatalytic activity. J. Saudi Chem. Soc. 2018, 22, 439–448. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, S.; Yang, D.; Zhai, H.; You, Y.; Fu, X.; Yuan, J.; Zhou, X.; Wen, J.; Liu, Y. Synthesis of X-architecture CeO2 for the photodegradation of methylene blue under UV-light irradiation. J. Alloy. Compd. 2017, 705, 131–137. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, W.; Peng, H.; Wen, J. Preparation and characterization of CuxZn1-xS nanodisks for the efficient visible light photocatalytic activity. J. Environ. Chem. Eng. 2018, 6, 9–18. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; You, Y.; Fu, X.; Liu, Y.; Wen, J. One-pot synthesis of sandwich-like MgO@Carbon with enhanced sorption capacity of organic dye. Chem. Eng. J. 2018, 334, 1399–1409. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Li, M.; Shi, Y.; Tang, Y. N-doped carbon dots/CdS hybrid photocatalyst that responds to visible/near-infrared light irradiation for enhanced photocatalytic hydrogen production. Sep. Purif. Technol. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Ling, H.; Qiu, Y.; Lou, J.; Hou, X.; Bag, S.; Wang, J.; Wu, H.; Chai, G. Significant enhancement of the visible light photocatalytic properties in 3D BiFeO3/graphene composites. Nanomaterials 2019, 9, 65. [Google Scholar] [CrossRef]
- Baran, T.; Dibenedetto, A.; Aresta, M.; Kruczala, K.; Macyk, W. Photocatalytic carboxylation of organic substrates with carbon dioxide at zinc sulfide with deposited ruthenium nanoparticles. ChemPlusChem 2014, 79, 708–715. [Google Scholar] [CrossRef]
- Yoon, H.J.; Choi, Y.I.; Jang, E.S.; Sohn, Y. Graphene, charcoal, ZnO, and ZnS/BiOX (X=Cl, Br, and I) hybrid microspheres for photocatalytic simulated real mixed dye treatments. J. Ind. Eng. Chem. 2015, 32, 137–152. [Google Scholar] [CrossRef]
- Choi, Y.I.; Lee, S.; Kim, S.K.; Kim, Y.; Cho, D.W.; Khan, M.M.; Sohn, Y. Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS microspheres for photocatalytic activities, CO oxidation and 2-hydroxyterephthalic acid synthesis. J. Alloy. Compd. 2016, 675, 46–56. [Google Scholar] [CrossRef]
- Pang, H.; Meng, X.; Song, H.; Zhou, W.; Yang, G.; Zhang, H.; Izumi, Y.; Takei, T.; Jewasuwan, W.; Fukata, N.; et al. Probing the role of nickel dopant in aqueous colloidal ZnS nanocrystals for efficient solar-driven CO2 reduction. Appl. Catal. B Environ. 2019, 244, 1013–1020. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Chen, M.; Li, W.; Yuan, Z.; Snyders, R. Visible-light-driven photocatalytic activities of monodisperse ZnS-coated reduced graphene oxide nanocomposites. Mater. Chem. Phys. 2019, 227, 368–374. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, Q.; Liu, Y.; Zhang, S.; Guo, J. Preparation of TiO2 and Fe-TiO2 with an impinging stream-rotating packed bed by the precipitation method for the photodegradation of gaseous toluene. Nanomaterials 2019, 9, 1173. [Google Scholar] [CrossRef]
- Negrin-Montecelo, Y.; Testa-Anta, M.; Marin-Caba, L.; Perez-Lorenzo, M.; Salgueirino, V.; Correa-Duarte, M.A.; Comesana-Hermo, M. Titanate nanowires as one-dimensional hot spot generators for broadband Au-TiO2 photocatalysis. Nanomaterials 2019, 9, 990. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Xiao, X.F.; Jung, H.S.; Giannini, V.; Maier, S.A.; Kim, D.H.; Lee, Y.I.; Park, S.G. Compact integration of TiO2 nanoparticles into the cross-points of 3D vertically stacked Ag nanowires for plasmon-enhanced photocatalysis. Nanomaterials 2019, 9, 468. [Google Scholar] [CrossRef]
- Radzig, M.; Koksharova, O.; Khmel, I.; Ivanov, V.; Yorov, K.; Kiwi, J.; Rtimi, S.; Tastekova, E.; Aybush, A.; Nadtochenko, V. Femtosecond spectroscopy of Au hot-electron injection into TiO2: Evidence for Au/TiO2 plasmon photocatalysis by bactericidal Au ions and related phenomena. Nanomaterials 2019, 9, 217. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Fan, B.; Xing, A.; Jia, B. Ferroelectric polarization-enhanced photocatalysis in BaTiO3-TiO2 core-shell heterostructures. Nanomaterials 2019, 9, 1116. [Google Scholar] [CrossRef]






| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| ZnS | 72.82 | 0.58 | 6.58 |
| ZnS@N-C-1 | 39.95 | 0.47 | 9.95 |
| ZnS@N-C-2 | 42.83 | 0.54 | 9.67 |
| ZnS@N-C-3 | 46.46 | 0.61 | 9.55 |
| ZnS@N-C-4 | 45.57 | 0.58 | 9.61 |
| ZnS@N-C-5 | 43.58 | 0.49 | 9.56 |
| Material | Dosage (mg) | Light Source | Pollutant | Time (min) | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| α-Fe2O3@TiO2 | 100 | Xe (300 W) | Tetracycline (50 mg L−1, 200 mL) | 90 | 100 | [4] |
| MgO@N-C | 100 | Xe (300 W) | Methylene blue (120 mg L−1, 100 mL) | 70 | 98.67 | [22] |
| N-C/g-C3N4 | 50 | Xe (350 W) | Indomethacin (4 mg L−1, 50 mL) | 90 | 91.75 | [28] |
| ZnS@N/S-C | 20 | Xe (300 W) | Bisphenol-A (200 mg L−1, 50 mL) | 200 | 88.00 | [31] |
| ZnS@N-C | 50 | Hg (150 W) | Methylene blue (10 mg L−1, 50 mL) | 110 | 97.20 | [32] |
| Sm2O3@N-C | 100 | Xe (300 W) | Tetracycline (50 mg L−1, 100 mL) | 120 | 90.26 | [34] |
| ZnS-15%RGO | 20 | Xe (300 W) | Methylene blue (20 mg L−1, 100 mL) | 240 | 89.43 | [55] |
| ZnS@N-C-3 | 100 | Xe (300 W) | Tetracycline (40 mg L−1, 100 mL) | 90 | 98.60 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Liu, D.; Zheng, X.; Fu, X. N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region. Nanomaterials 2019, 9, 1657. https://doi.org/10.3390/nano9121657
Peng H, Liu D, Zheng X, Fu X. N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region. Nanomaterials. 2019; 9(12):1657. https://doi.org/10.3390/nano9121657
Chicago/Turabian StylePeng, Hao, Daixin Liu, Xiaogang Zheng, and Xiaojin Fu. 2019. "N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region" Nanomaterials 9, no. 12: 1657. https://doi.org/10.3390/nano9121657
APA StylePeng, H., Liu, D., Zheng, X., & Fu, X. (2019). N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region. Nanomaterials, 9(12), 1657. https://doi.org/10.3390/nano9121657







