All-Inorganic Perovskite Solar Cells Based on CsPbIBr2 and Metal Oxide Transport Layers with Improved Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Nanoparticles Preparation and Device Fabrication
2.2.1. Preparation of NiOx Nanoparticles
2.2.2. Preparation of Precursor Solutions of CeOx and CsPbIBr2
2.2.3. Device Fabrication
2.3. Characterization
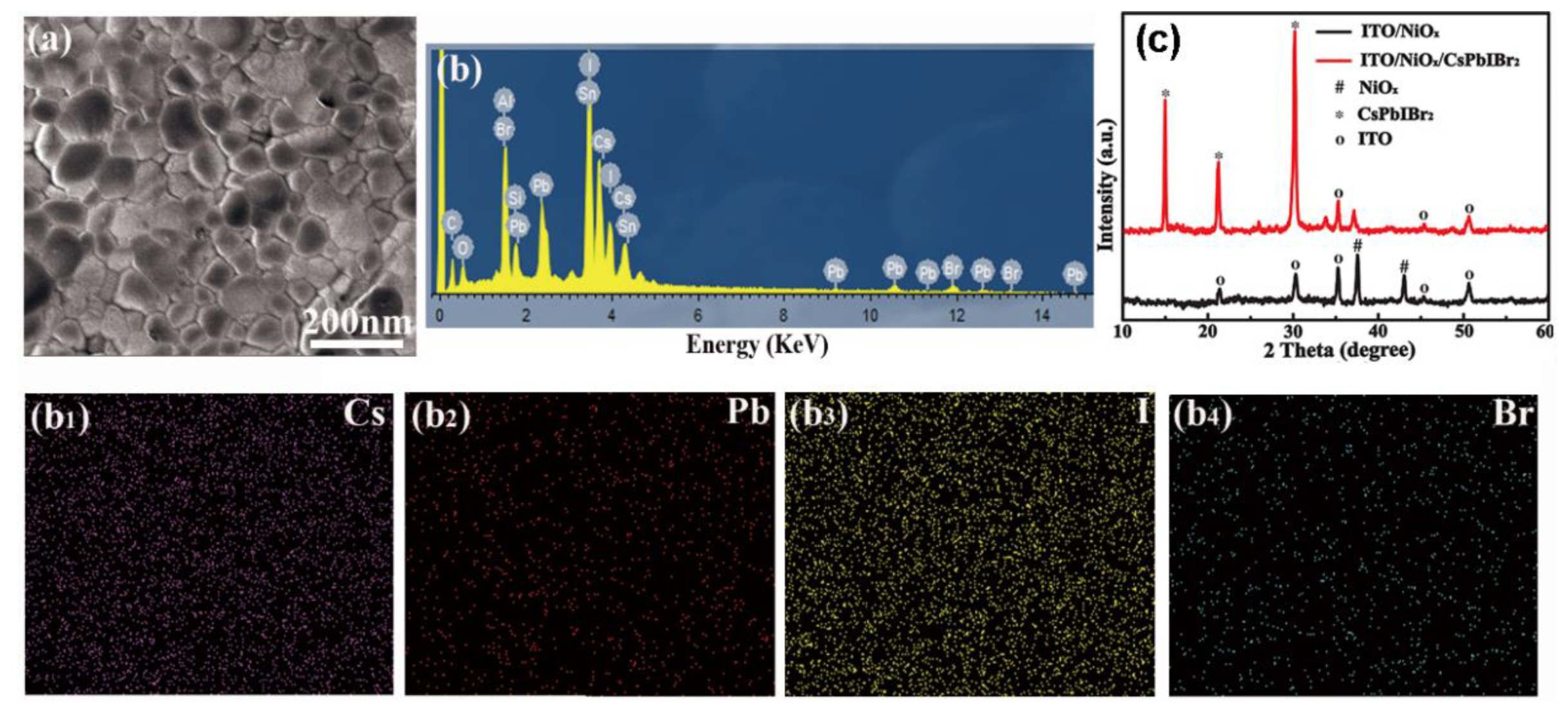
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NREL. Best Research Efficiencies. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 June 2019).
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 Degradation Rates and Mechanisms in Controlled Humidity Environments Using in Situ Techniques. ACS Nano 2015, 9, 1955–1963. [Google Scholar] [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Cesium lead halide perovskites with improved stability for tandem solar cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kang, G.; Malekshahi Byranvand, M.; Lee, G.-Y.; Park, T. Gradated mixed hole transport layer in a perovskite solar cell: Improving moisture stability and efficiency. ACS Appl. Mater. Interfaces 2017, 9, 27720–27726. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X= Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Xing, X.; Jiang, Y.; He, T.; Huang, Y.; Ma, Z.; Yang, J.; Yuan, M. Conjugated Alkylamine by Two-Step Surface Ligand Engineering in CsPbBr3 Perovskite Nanocrystals for Efficient Light-Emitting Diodes. ChemNanoMat 2018, 5, 318–322. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Li, H.; Wang, H.; Zhang, C.; Yang, Y.; Gao, X.; Xue, Q.; Yip, H.-L.; Fan, J.; et al. Structurally Reconstructed CsPbI2Br Perovskite for Highly Stable and Square-Centimeter All-Inorganic Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803572. [Google Scholar] [CrossRef]
- Ma, J.; Chang, J.; Lin, Z.; Guo, X.; Zhou, L.; Liu, Z.; Xi, H.; Chen, D.; Zhang, C.; Hao, Y. Elucidating the roles of TiCl4 and PCBM fullerene treatment on TiO2 electron transporting layer for highly efficient planar perovskite solar cells. J. Phys. Chem. C 2018, 122, 1044–1053. [Google Scholar] [CrossRef]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Nejand, B.A.; Moshaii, A.; Mohammadian, N.; Alizadeh, A.H.; Mohammadpour, R.; Ahmadi, V.; Alizadeh, A. Two-step physical deposition of a compact CuI Hole-Transport layer and the formation of an interfacial species in perovskite solar cells. ChemSusChem 2016, 9, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Kim, H.; Kim, H.H.; Shim, S.E.; Hong, C.K. Nanoporous p-type NiOx electrode for pin inverted perovskite solar cell toward air stability. Mater. Today 2018, 21, 483–500. [Google Scholar] [CrossRef]
- Ahmad, S.; Fu, P.; Yu, S.; Yang, Q.; Liu, X.; Wang, X.; Wang, X.; Guo, X.; Li, C. Dion-Jacobson Phase 2D Layered Perovskites for Solar Cells with Ultrahigh Stability. Joule 2019, 3, 794–806. [Google Scholar] [CrossRef]
- Hwang, I.; Yong, K. Novel CdS hole-blocking layer for photostable perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 4226–4232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, J.; Ni, Y.; Yang, J.; Wang, Y.; Jiu, T.; Yuan, M.; Chen, J. Reduced-dimensional α-CsPbX3 perovskites for efficient and stable photovoltaics. Joule 2018, 2, 1356–1368. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, S.; Wen, X.; Green, M.A.; Ho-Baillie, A.W.Y. Hole Transport Layer Free Inorganic CsPbIBr2 Perovskite Solar Cell by Dual Source Thermal Evaporation. Adv. Energy Mater. 2016, 6, 1502202. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Wang, X.; Liu, S. Interface-Modification-Induced Gradient Energy Band for Highly Efficient CsPbIBr2 Perovskite Solar Cells. Adv. Energy Mater. 2019. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef]
- Wang, X.; Deng, L.-L.; Wang, L.-Y.; Dai, S.-M.; Xing, Z.; Zhan, X.-X.; Lu, X.-Z.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Cerium oxide standing out as an electron transport layer for efficient and stable perovskite solar cells processed at low temperature. J. Mater. Chem. A 2017, 5, 1706–1712. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Wu, B.-S.; Wang, X.; Wang, L.-Y.; Wang, T.; Liu, H.-R.; Zhang, M.-L.; Yun, D.-Q.; Deng, L.-L.; et al. Photovoltaic performance and stability of fullerene/cerium oxide double electron transport layer superior to single one in p-i-n perovskite solar cells. J. Power Sources 2018, 389, 13–19. [Google Scholar] [CrossRef]
- Woo, K.; Kim, Y.; Moon, J. A non-toxic, solution-processed, earth abundant absorbing layer for thin-film solar cells. Energy Environ. Sci. 2012, 5, 5340–5345. [Google Scholar] [CrossRef]
- Lu, J.; Chen, S.-C.; Zheng, Q. Defect Passivation of CsPbIBr2 Perovskites for High-Performance Solar Cells with Large Open-Circuit Voltage of 1. 28 V. ACS Appl. Energy Mater. 2018, 1, 5872–5878. [Google Scholar] [CrossRef]
- Shrotriya, V.; Li, G.; Yao, Y.; Chu, C.W.; Yang, Y. Transition metal oxides as the buffer layer for polymer photovoltaic cells. Appl. Phys. Lett. 2006, 88, 073508. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Shuang, X.; Xue, Q.; Teng, Z.; Zhu, Z.; Qiang, L.; Chen, H.; Yun, Y.; Hu, Z. Effects of a Molecular Monolayer Modification of NiO Nanocrystal Layer Surfaces on Perovskite Crystallization and Interface Contact toward Faster Hole Extraction and Higher Photovoltaic Performance. Adv. Funct. Mater. 2016, 26, 2950–2958. [Google Scholar]
- Liu, Z.; Zhu, A.; Cai, F.; Tao, L.; Zhou, Y.; Zhao, Z.; Qi, C.; Cheng, Y.B.; Zhou, H. Nickel oxide nanoparticles for efficient hole transport in p-i-n and n-i-p perovskite solar cells. J. Mater. Chem. A 2017, 5, 6597–6605. [Google Scholar] [CrossRef]
- Dong, Y.; Xin, Z.; Yang, R.; Zhou, Y.; Wei, Y.; Wang, X.; Li, C.; Liu, S.F.; Chang, R. Surface Optimization to Eliminate Hysteresis for Record Efficiency Planar Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Yun, S.-C.; Ma, S.; Kwon, H.-C.; Kim, K.; Jang, G.; Yang, H.; Moon, J. Amino Acid Salt-Driven Planar Hybrid Perovskite Solar Cells With Enhanced Humidity Stability. Nano Energy 2019, 59, 481–491. [Google Scholar] [CrossRef]
- Zhu, W.; Shen, C.; Wu, Y.; Zhang, H.; Li, E.; Zhang, W.; Xu, X.; Wu, W.; Tian, H. Semi-Locked Tetrathienylethene as Promising Building Block for Hole Transporting Materials: Toward Efficient and Stable Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 3784–3789. [Google Scholar]
- Cheng, R.; Chung, C.C.; Zhang, H.; Zhou, Z.; Zhai, P.; Huang, Y.T.; Lee, H.; Feng, S.P. An Air Knife–Assisted Recrystallization Method for Ambient-Process Planar Perovskite Solar Cells and Its Dim-Light Harvesting. Small 2019, 15, e1804465. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Xu, J.; Zhang, Q.; Liu, H.; Liu, Z.; Yuan, M. A Review on Improving the Quality of Perovskite Films in Perovskite Solar Cells via the Weak Forces Induced by Additives. Appl. Sci. 2019, 9, 4393. [Google Scholar] [CrossRef] [Green Version]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, Q.; Xu, J.; Liu, H.; Qin, R.; Zhai, H.; Chen, S.; Yuan, M. All-Inorganic Perovskite Solar Cells Based on CsPbIBr2 and Metal Oxide Transport Layers with Improved Stability. Nanomaterials 2019, 9, 1666. https://doi.org/10.3390/nano9121666
Yang J, Zhang Q, Xu J, Liu H, Qin R, Zhai H, Chen S, Yuan M. All-Inorganic Perovskite Solar Cells Based on CsPbIBr2 and Metal Oxide Transport Layers with Improved Stability. Nanomaterials. 2019; 9(12):1666. https://doi.org/10.3390/nano9121666
Chicago/Turabian StyleYang, Jien, Qiong Zhang, Jinjin Xu, Hairui Liu, Ruiping Qin, Haifa Zhai, Songhua Chen, and Mingjian Yuan. 2019. "All-Inorganic Perovskite Solar Cells Based on CsPbIBr2 and Metal Oxide Transport Layers with Improved Stability" Nanomaterials 9, no. 12: 1666. https://doi.org/10.3390/nano9121666




