Increased Flexibility in Lab-on-Chip Design with a Polymer Patchwork Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication with the Patchwork Approach
2.2. Fabrication of Control Devices
2.3. Optical and Atomic Force Microscopy (AFM) Characterization of the Device
2.4. Electrical Measurements
3. Results and Discussion
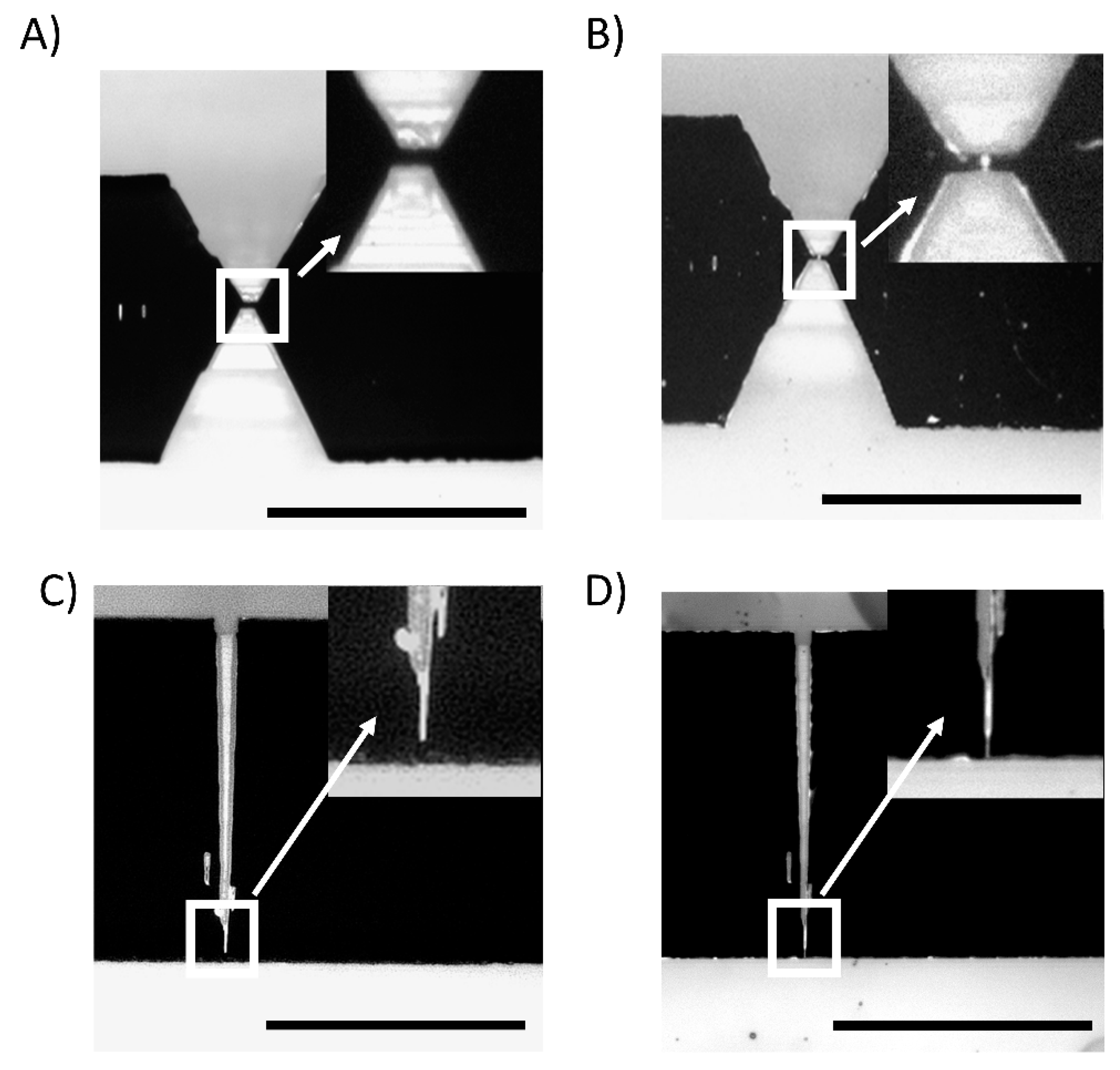
3.1. AFM-Based Analysis of the Dimensions of the Casts
3.2. Electrical Characterization of Devices
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Yang, J.; Cai, Z.; Feng, Y.; Wang, Y.; Zhang, D.; Pan, X. Detection of engineered nanoparticles in aquatic environments: Current status and challenges in enrichment, separation, and analysis. Environ. Sci. Nano 2019, 6, 709–735. [Google Scholar] [CrossRef]
- Nie, X.; Liu, H.; Pan, Z.; Ahmed, S.; Shen, Q.; Yang, J.; Pan, J.; Pang, J.; Li, C.; Xia, X.; et al. Recognition of plastic nanoparticles using a single gold nanopore fabricated at the tip of a glass nanopipette. Chem. Commun. 2019, 55, 6397–6400. [Google Scholar] [CrossRef]
- Wei, R.; Gatterdam, V.; Wieneke, R.; Tampe, R.; Rant, U. Stochastic sensing of proteins with receptor-modified solid-state nanopores. Nat. Nanotechnol. 2012, 7, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Creus, M.; Schurmann, G.; Linder, V.; Ward, T.; de Rooij, N.; Staufer, U. Label-free detection of single protein molecules and protein-protein interactions using synthetic nanopores. Anal. Chem. 2008, 80, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Fanzio, P.; Mussi, V.; Menotta, M.; Firpo, G.; Repetto, L.; Guida, P.; Angeli, E.; Magnani, M.; Valbusa, U. Selective protein detection with a dsLNA-functionalized nanopore. Biosens. Bioelectron. 2015, 64, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Harms, Z.; Mogensen, K.; Nunes, P.; Zhou, K.; Hildenbrand, B.; Mitra, I.; Tan, Z.; Zlotnick, A.; Kutter, J.; Jacobson, S. Nanofluidic Devices with Two Pores in Series for Resistive-Pulse Sensing of Single Virus Capsids. Anal. Chem. 2011, 83, 9573–9578. [Google Scholar] [CrossRef]
- Mitra, A.; Deutsch, B.; Ignatovich, F.; Dykes, C.; Novotny, L. Nano-optofluidic Detection of Single Viruses and Nanoparticles. Acs Nano 2010, 4, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yamamoto, T. Quantification of Virus Particles Using Nanopore-Based Resistive-Pulse Sensing Techniques. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Aizel, K.; Agache, V.; Pudda, C.; Bottausci, F.; Fraisseix, C.; Bruniaux, J.; Navarro, F.; Fouillet, Y. Enrichment of nanoparticles and bacteria using electroless and manual actuation modes of a bypass nanofluidic device. Lab Chip 2013, 13, 4476–4485. [Google Scholar] [CrossRef]
- Wang, Z.; Han, T.; Jeon, T.; Park, S.; Kim, S. Rapid detection and quantification of bacteria using an integrated micro/nanofluidic device. Sens. Actuators B Chem. 2013, 178, 683–688. [Google Scholar] [CrossRef]
- Fan, R.; Karnik, R.; Yue, M.; Li, D.; Majumdar, A.; Yang, P. DNA translocation in inorganic nanotubes. Nano Lett. 2005, 5, 1633–1637. [Google Scholar] [CrossRef]
- Mussi, V.; Fanzio, P.; Repetto, L.; Firpo, G.; Scaruffi, P.; Stigliani, S.; Tonini, G.; Valbusa, U. DNA-functionalized solid state nanopore for biosensing. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Menard, L.; Ramsey, J. Electrokinetically-Driven Transport of DNA through Focused Ion Beam Milled Nanofluidic Channels. Anal. Chem. 2013, 85, 1146–1153. [Google Scholar] [CrossRef]
- Piruska, A.; Gong, M.; Sweedler, J.; Bohn, P. Nanofluidics in chemical analysis. Chem. Soc. Rev. 2010, 39, 1060–1072. [Google Scholar] [CrossRef]
- Majd, S.; Yusko, E.; Billeh, Y.; Macrae, M.; Yang, J.; Mayer, M. Applications of biological pores in nanomedicine, sensing, and nanoelectronics. Curr. Opin. Biotechnol. 2010, 21, 439–476. [Google Scholar] [CrossRef]
- Venkatesan, B.; Bashir, R. Nanopore sensors for nucleic acid analysis. Nat. Nanotechnol. 2011, 6, 615–624. [Google Scholar] [CrossRef]
- Kim, M.; McNally, B.; Murata, K.; Meller, A. Characteristics of solid-state nanometre pores fabricated using a transmission electron microscope. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Hinkle, P.; Westerhof, T.; Qiu, Y.; Mallin, D.; Wallace, M.; Nelson, E.; Taborek, P.; Siwy, Z. A hybrid resistive pulse-optical detection platform for microfluidic experiments. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Pedone, D.; Langecker, M.; Munzer, A.; Wei, R.; Nagel, R.; Rant, U. Fabrication and electrical characterization of a pore-cavity-pore device. J. Phys. Condens. Matter 2010, 22. [Google Scholar] [CrossRef]
- Gates, B.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.; Whitesides, G. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef]
- Lo, C.; Aref, T.; Bezryadin, A. Fabrication of symmetric sub-5 nm nanopores using focused ion and electron beams. Nanotechnology 2006, 17, 3264–3267. [Google Scholar] [CrossRef]
- Tseng, A. Recent developments in micromilling using focused ion beam technology. J. Micromec. Microeng. 2004, 14, R15–R34. [Google Scholar] [CrossRef]
- Altissimo, M. E-beam lithography for micro-/nanofabrication. Biomicrofluidics 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cheng, Y.; Liu, C.; Song, J.; He, F.; Shen, Y.; Chen, D.; Xu, Z.; Fan, Z.; Wei, X.; et al. Direct laser writing of sub-50 nm nanofluidic channels buried in glass for three-dimensional micro-nanofluidic integration. Lab Chip 2013, 13, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.P.; Qin, S.J.; Li, W.J.; Wang, M.Y. High aspect ratio nano fluidic channels by laser-controlled fracturing. In Proceedings of the Fifteenth IEEE International Conference on Micro Electro Mechanical Systems, Las Vegas, NV, USA, 24 January 2002; pp. 156–159. [Google Scholar] [CrossRef]
- Chantiwas, R.; Hupert, M.; Pullagurla, S.; Balamurugan, S.; Tamarit-Lopez, J.; Park, S.; Datta, P.; Goettert, J.; Cho, Y.; Soper, S. Simple replication methods for producing nanoslits in thermoplastics and the transport dynamics of double-stranded DNA through these slits. Lab Chip 2010, 10, 3255–3264. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Duffy, D.; Anderson, J.; Chiu, D.; Wu, H.; Schueller, O.; Whitesides, G. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Manneschi, C.; Fanzio, P.; Ala-Nissila, T.; Angeli, E.; Repetto, L.; Firpo, G.; Valbusa, U. Stretching of DNA confined in nanochannels with charged walls. Biomicrofluidics 2014, 8. [Google Scholar] [CrossRef]
- Odom, T.; Love, J.; Wolfe, D.; Paul, K.; Whitesides, G. Improved pattern transfer in soft lithography using composite stamps. Langmuir 2002, 18, 5314–5320. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, Y.; Menard, E.; Aluru, N.; Rogers, J.; Alleyne, A. Mechanism for stamp collapse in soft lithography. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, W.; Hsia, K.; Menard, E.; Park, J.; Rogers, J.; Alleyne, A. Stamp collapse in soft lithography. Langmuir 2005, 21, 8058–8068. [Google Scholar] [CrossRef]
- Schmid, H.; Michel, B. Siloxane polymers for high-resolution, high-accuracy soft lithography. Macromolecules 2000, 33, 3042–3049. [Google Scholar] [CrossRef]
- Choi, K.; Rogers, J. A photocurable poly(dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. J. Am. Chem. Soc. 2003, 125, 4060–4061. [Google Scholar] [CrossRef] [PubMed]
- Huelsen, C.; Probst, J.; Loechel, B. Replication of sub-100 nm structures using h- and s-PDMS composite stamps. Microsyst. Technol. 2014, 20, 2001–2004. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Song, N.; Choo, B.; Pribat, D.; Jang, J.; Park, K.; Yoo, S. Mechanical characteristics of the hard-polydimethylsiloxane for smart lithography. Ekc2008 Proc. EU-Korea Conf. Sci. Technol. 2008, 124, 229–237. [Google Scholar]
- Huh, D.; Mills, K.; Zhu, X.; Burns, M.; Thouless, M.; Takayama, S. Tuneable elastomeric nanochannels for nanofluidic manipulation. Nat. Mater. 2007, 6, 424–428. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78. [Google Scholar] [CrossRef]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Mao, P.; Han, J. Fabrication and characterization of 20 nm planar nanofluidic channels by glass-glass and glass-silicon bonding. Lab Chip 2005, 5, 837–844. [Google Scholar] [CrossRef]
- Pinti, M.; Prakash, S. Fabrication of Hybrid Micro-Nanofluidic Devices with Centimeter Long Ultra-Low Aspect Ratio Nanochannels. In Proceedings of the Asme International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013; Volume 10. [Google Scholar] [CrossRef]
- Hou, X.; Dong, H.; Zhu, D.; Jiang, L. Fabrication of Stable Single Nanochannels with Controllable Ionic Rectification. Small 2010, 6, 361–365. [Google Scholar] [CrossRef]
- Angeli, E.; Volpe, A.; Fanzio, P.; Repetto, L.; Firpo, G.; Guida, P.; Lo Savio, R.; Wanunu, M.; Valbusa, U. Simultaneous Electro-Optical Tracking for Nanoparticle Recognition and Counting. Nano Lett. 2015, 15, 5696–5701. [Google Scholar] [CrossRef] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzuoli, D.; Angeli, E.; Repetto, D.; Guida, P.; Firpo, G.; Repetto, L. Increased Flexibility in Lab-on-Chip Design with a Polymer Patchwork Approach. Nanomaterials 2019, 9, 1678. https://doi.org/10.3390/nano9121678
Pezzuoli D, Angeli E, Repetto D, Guida P, Firpo G, Repetto L. Increased Flexibility in Lab-on-Chip Design with a Polymer Patchwork Approach. Nanomaterials. 2019; 9(12):1678. https://doi.org/10.3390/nano9121678
Chicago/Turabian StylePezzuoli, Denise, Elena Angeli, Diego Repetto, Patrizia Guida, Giuseppe Firpo, and Luca Repetto. 2019. "Increased Flexibility in Lab-on-Chip Design with a Polymer Patchwork Approach" Nanomaterials 9, no. 12: 1678. https://doi.org/10.3390/nano9121678
APA StylePezzuoli, D., Angeli, E., Repetto, D., Guida, P., Firpo, G., & Repetto, L. (2019). Increased Flexibility in Lab-on-Chip Design with a Polymer Patchwork Approach. Nanomaterials, 9(12), 1678. https://doi.org/10.3390/nano9121678





