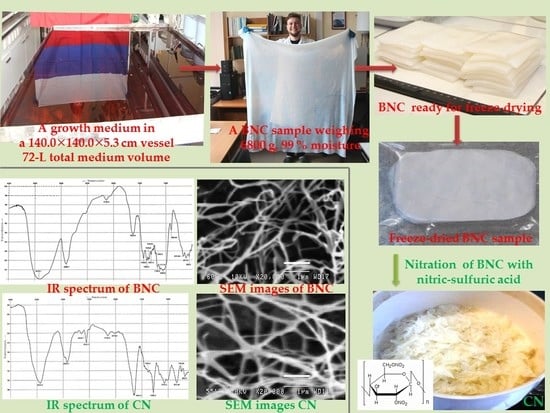
Bacterial Nanocellulose Nitrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biosynthesis of BNC
2.1.1. BNC-Producing Symbiont
2.1.2. Synthesis of BNC Gel-Films
2.1.3. Washing of BNC
2.1.4. Calculation of BNC Yield
2.1.5. Quality Attributes of BNC
2.1.6. Examination of BNC Structure
2.1.7. Freeze-Drying of BNC
2.2. Nitration of BNC
2.2.1. Preparation of BNC for Nitration
2.2.2. Nitration and Stabilization of BNC
2.2.3. Calculation of CN Yield
2.2.4. Analysis of CN
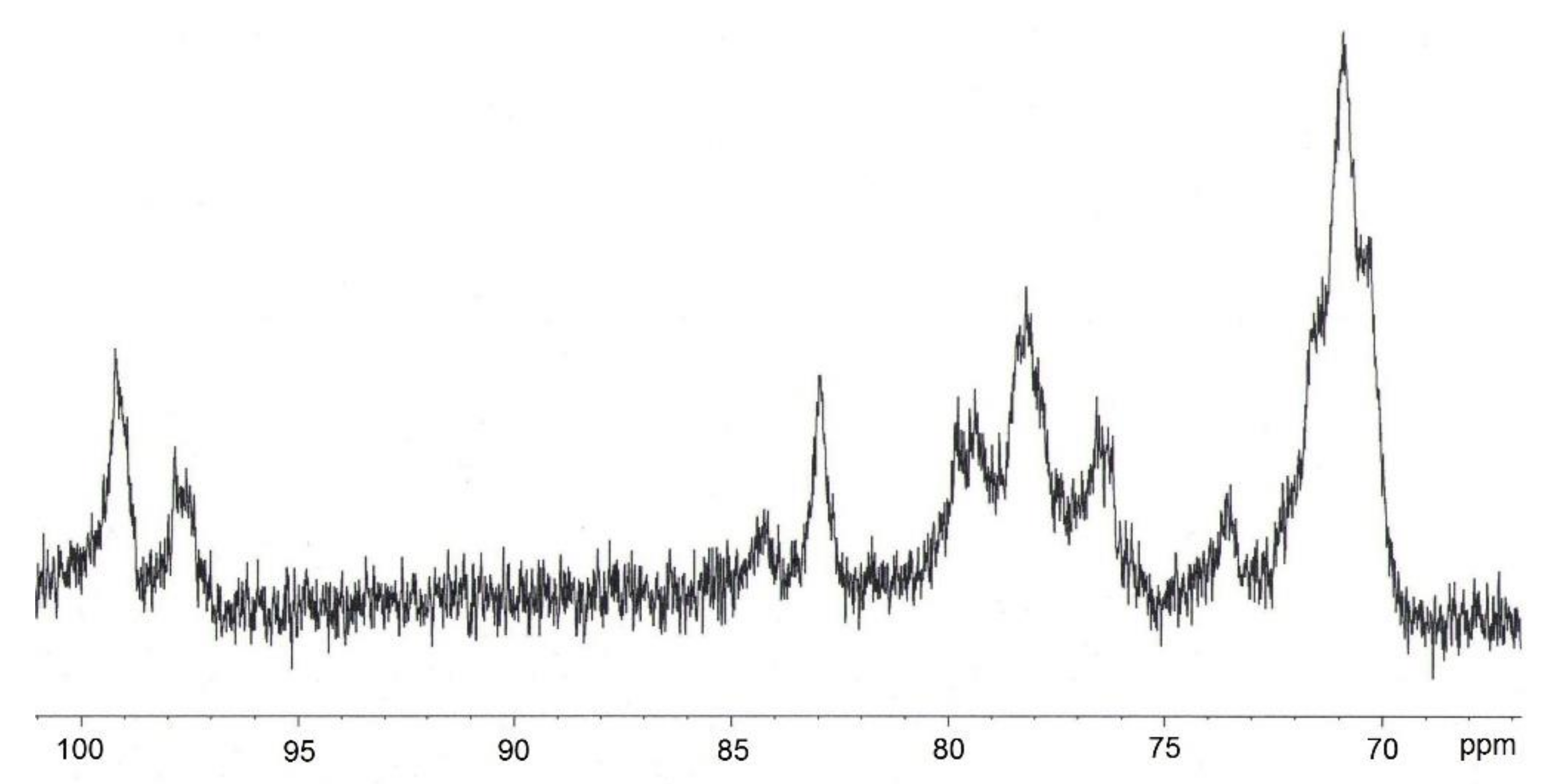
2.2.5. Examination of CN Structure
3. Results and Discussion
3.1. Biosynthesis of BNC
3.2. Nitration of BNC
3.3. Analysis Results for BNC and CN Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gama, M.; Dourado, F.; Bielecki, S. (Eds.) Bacterial Nanocellulose: From Biotechnology to Bio-Economy; Elsevier: Oxford, UK, 2016; p. 260. [Google Scholar]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2014, 22, 565–579. [Google Scholar] [CrossRef]
- Moniri, M.; Moghaddam, A.B.; Azizi, S.; Rahim, R.A.; Bin Ariff, A.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Wu, Y.; He, K.; Li, Y.; Luo, X.; Li, B.; Wang, C.; Liu, S. Flexible cellulose nanofibrils as novel pickering stabilizers: The emulsifying property and packing behavior. Food Hydrocoll. 2019, 88, 180–189. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Bai, Y.-L.; Liu, X.; Ma, C.; Wu, X.-Y.; Wei, X.; Wang, Z.; Wang, K.-X.; Chen, J.-S. Free-Standing Hybrid Porous Membranes Integrated with Transition Metal Nitride and Carbide Nanoparticles for High-Performance Lithium-Sulfur Batteries. Chem. Eng. J. 2019, 378, 122208. [Google Scholar] [CrossRef]
- Shim, E.; Su, J.; Noro, J.; Teixeira, M.A.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Conductive bacterial cellulose by in situ laccase polymerization of aniline. PLoS ONE 2019, 14, e0214546. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Luo, Q.; Zhu, J.; Li, Z.; Duan, X.; Pei, C.; Mao, C. The Solution Characteristics of Nitrated Bacterial Cellulose in Acetone. New J. Chem. 2018, 42, 18252–18258. [Google Scholar] [CrossRef]
- Chai, H.; Duan, Q.; Jiang, L.; Gong, L.; Chen, H.; Sun, J. Theoretical and experimental study on the effect of nitrogen content on the thermal characteristics of nitrocellulose under low heating rates. Cellulose 2019, 26, 763–776. [Google Scholar] [CrossRef]
- Nikolsky, S.N.; Zlenko, D.V.; Melnikov, V.P.; Stovbun, S.V. The fibrils untwisting limits the rate of cellulose nitration process. Carbohydr. Polym. 2019, 204, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Horii, F.; Hirai, A. Structural studies of bacterial cellulose through the solid-phase nitration and acetylation by CP/MAS 13C NMR spectroscopy. Cellulose 2006, 13, 327–342. [Google Scholar] [CrossRef]
- Sun, D.-P.; Ma, B.; Zhu, C.-L.; Liu, C.-S.; Yang, J.-Z. Novel nitrocellulose made from bacterial cellulose. J. Energ. Mater. 2010, 28, 85–97. [Google Scholar] [CrossRef]
- Peters, G.; Endsor, R.; Hamid, J.; Claridge, R.; Arber, A.; Dennis, C. Nitration of Bacterial Cellulose. In Proceedings of the 5th International Nitrocellulose Symposium, Spiez, Switzerland, 17–18 April 2012; p. 40. [Google Scholar]
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]
- Gomes, F.P.; Silva, N.H.; Trovatti, E.; Serafim, L.S.; Duarte, M.F.; Silvestre, A.J.; Freire, C.S. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass Bioenerg. 2013, 55, 205–211. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors affecting the yield and properties of bacterial cellulose. J. Ind. Microbiol. Biot. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal Compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Kutyshenko, V.P.; Yurkevich, D.I. Influence of heavy water on the metabolism of a symbiotic organism. Biophysics 2003, 48, 648–656. [Google Scholar]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Aleshina, L.A.; Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Arkharova, N.A.; Sakovich, G.V. X-ray Diffraction Study of Bacterial Nanocellulose Produced by the Medusomyces Gisevii Sa-12 Culture in Enzymatic Hydrolysates of Oat Hulls. Crystallogr. Rep. 2018, 63, 955–960. [Google Scholar] [CrossRef]
- Aleshina, L.A.; Gladysheva, E.K.; Budaeva, V.V.; Golubev, D.S.; Skiba, E.A.; Sakovich, G.V. X-ray Diffraction Study of Bacterial Nanocellulose Produced by Medusomyces Gisevii Sa-12 Cultured in Enzymatic Hydrolysates of Miscanthus. Crystallogr. Rep. 2019, 64, 914–919. [Google Scholar] [CrossRef]
- Gladysheva, E.K.; Skiba, E.A.; Zolotukhin, V.N.; Sakovich, G.V. Study of the Conditions for the Biosynthesis of Bacterial Cellulose by the Producer Medusomyces gisevii Sa-12. Appl. Biochem. Microbiol. 2018, 54, 179–187. [Google Scholar] [CrossRef]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Rajeev, B. Fermentation of black tea broth (Kombucha): I. Effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohydr. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- McKenna, B.A.; Mikkelsen, D.; Wehr, J.B.; Gidley, M.J.; Menzies, N.W. Mechanical and structural properties of native and alkalitreated bacterial cellulose produced by Gluconacetobacter xylinus strain ATCC 53524. Cellulose 2009, 16, 1047–1055. [Google Scholar] [CrossRef]
- Younesi, M.; Wu, X.; Akkus, O. Controlled mercerization of bacterial cellulose provides tunability of modulus and ductility over two orders of magnitude. J. Mech. Behav. Biomed. Mater. 2019, 90, 530–537. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum: II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Chandrasekaran, P.T.; Bari, N.K.; Sinha, S. Enhanced bacterial cellulose production from Gluconobacter xylinus using super optimal broth. Cellulose 2017, 24, 4367–4381. [Google Scholar] [CrossRef]
- Obolenskaya, A.V.; Yelnitskaya, Z.P.; Leonovich, A.A. Laboratornye Raboty po Khimii Drevesiny I Tsellyulozy [Laboratory Works on Wood and Cellulose Chemistry]: Textbook for Higher Educational Institutions; Ecology Publisher: Moscow, Russia, 1991; p. 72. (In Russian) [Google Scholar]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Novyi Spravochnik Khimika i Tekhnologa. Syr’e i Produkty Promyshlennosti Organicheskikh i Neorganicheskikh Veshchestv; [New Reference Book for Chemist and Engineer. Feedstock and Products of Organic and Inorganic Industry]; NPO Professional Publisher: St. Petersburg, Russia, 2006; p. 1142. (In Russian) [Google Scholar]
- Gensh, K.V.; Kolosov, P.V.; Bazarnova, N.G. Quantitative analysis of cellulose nitrates by Fourier transform infrared spectroscopy. Russ. J. Bioorg. Chem. 2011, 37, 814–816. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose Synthesis from Miscanthus Cellulose. Propellants Explos. Pyrotech. 2018, 43, 96–100. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitric acid preparation of cellulose from miscanthus as a nitrocellulose precursor. Russ. Chem. Bull. 2015, 64, 2949–2953. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Cellulose nitrates from intermediate flax straw. Russ. Chem. Bull. 2016, 65, 2920–2924. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A. Prospects of cellulose nitrates from unconventional feedstocks for use in composite explosives. Khimiya Rastit. Syrya Chem. Plant Raw Mater. 2019, 1, 259–268. (In Russian) [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Sopin, V.F.; Khrapkovskii, G.M. Strukturno-Kineticheskie Osobennosti Polucheniia i Termodestruktsii Nitratov Tselliuloz [Structural and Kinetic Features of Preparation and Thermal Degradation of Cellulose Nitrate]; Nauka: Moscow, Russia, 2005; p. 213. (In Russian) [Google Scholar]
- Luo, L.; Jin, B.; Xiao, Y.; Zhang, Q.; Chai, Z.; Huang, Q.; Chu, S.; Peng, R. Study on the isothermal decomposition kinetics and mechanism of nitrocellulose. Polym. Test. 2019, 75, 337–343. [Google Scholar] [CrossRef]
- Moore, D.S.; McGrane, S.D. Comparative infrared and Raman spectroscopy of energetic polymers. J. Mol. Struct. 2003, 661–662, 561–566. [Google Scholar] [CrossRef]
- De la Ossa, M.Á.F.; López-López, M.; Torre, M.; García-Ruiz, C. Analytical techniques in the study of highly-nitrated nitrocellulose. Trac-Trend. Anal. Chem. 2011, 30, 1740–1755. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Marchenko, G.N.; Khrapkovskii, G.M.; Shamov, A.G. Stroenie, Sintez i Termicheskoe Razlozhenie Nitratov Cellyulozy [The Structure, Synthesis and Thermal Decomposition of Cellulose Nitrates]; KDU: Moscow, Russia, 2012; p. 396. (In Russian) [Google Scholar]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J. Therm. Anal. Calorim. 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Ovchinnikova, E.V.; Gladysheva, E.K.; Kashcheyeva, E.I.; Pavlov, I.N.; Sakovich, G.V. A technology for pilot production of bacterial cellulose from oat hulls. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]




| Culture Cycle | Culture Vessel Dimensions (cm × cm × cm) | Culture Vessel Volume (L) | Fullness Coefficient of Culture Vessel | Qnty of Vessels (pcs) | Total Volume of Nutrient Medium (L) | Thickness of Medium Layer (cm) |
|---|---|---|---|---|---|---|
| I | 49.5 × 69.5 × 5.0 | 17 | 0.59 | 1 | 10 | 2.9 |
| II | 36.5 × 36.5 × 8.3 | 11 | 0.59 | 3 | 19.5 | 4.9 |
| III | 140 × 140 × 5.3 | 104 | 0.69 | 1 | 72 | 3.7 |
| Culture Cycle | Total Volume of Medium (V, L) | Residual Volume of Medium (L) | Initial Glucose Concentration in Medium (Cg, g/L) | Residual Glucose Concentration in Medium (g/L) | BNC Gel-Film Weight (m, g) | BNC Yield (η, %) |
|---|---|---|---|---|---|---|
| I | 10.0 | 4.3 | 18.4 | 2.8 | 1074 | 6.4 |
| II | 19.5 | 11.9 | 18.2 | 6.2 | 1558 | 4.9 |
| III | 72.0 | 42.8 | 19.9 | 4.5 | 6800 | 5.2 |
| Sample Name | Nitrogen Content (%) | Viscosity of 2% CN-Acetone Solution (sP) | Solubility in the Alcohol-Ester Mixture (%) | Ash Content (%) |
|---|---|---|---|---|
| BNC-based CN | 10.96 | 916 | 47 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budaeva, V.V.; Gismatulina, Y.A.; Mironova, G.F.; Skiba, E.A.; Gladysheva, E.K.; Kashcheyeva, E.I.; Baibakova, O.V.; Korchagina, A.A.; Shavyrkina, N.A.; Golubev, D.S.; et al. Bacterial Nanocellulose Nitrates. Nanomaterials 2019, 9, 1694. https://doi.org/10.3390/nano9121694
Budaeva VV, Gismatulina YA, Mironova GF, Skiba EA, Gladysheva EK, Kashcheyeva EI, Baibakova OV, Korchagina AA, Shavyrkina NA, Golubev DS, et al. Bacterial Nanocellulose Nitrates. Nanomaterials. 2019; 9(12):1694. https://doi.org/10.3390/nano9121694
Chicago/Turabian StyleBudaeva, Vera V., Yulia A. Gismatulina, Galina F. Mironova, Ekaterina A. Skiba, Evgenia K. Gladysheva, Ekaterina I. Kashcheyeva, Olga V. Baibakova, Anna A. Korchagina, Nadezhda A. Shavyrkina, Dmitry S. Golubev, and et al. 2019. "Bacterial Nanocellulose Nitrates" Nanomaterials 9, no. 12: 1694. https://doi.org/10.3390/nano9121694
APA StyleBudaeva, V. V., Gismatulina, Y. A., Mironova, G. F., Skiba, E. A., Gladysheva, E. K., Kashcheyeva, E. I., Baibakova, O. V., Korchagina, A. A., Shavyrkina, N. A., Golubev, D. S., Bychin, N. V., Pavlov, I. N., & Sakovich, G. V. (2019). Bacterial Nanocellulose Nitrates. Nanomaterials, 9(12), 1694. https://doi.org/10.3390/nano9121694