1. Introduction
Barium titanate (BaTiO
3) has a typical perovskite structure which exists in a number of polymorphs from high to low temperature: hexagonal, cubic, tetragonal, orthorhombic, and rhombohedral crystal structure. All polymorphs are related to the cubic ABO
3 perovskite structure which consists of close-packed AO
3 layers stacked along [001] in a cubic sequence (i.e., ABCABC) with B cations occupying octahedral sites to form a lattice of corner-linked BO
6 octahedra [
1]. The tetragonal polymorph of BaTiO
3, in which the Ti
4+ cations are displaced from the centre of the octahedra towards an apex, has received much attention as a result of the high permittivity at the tetragonal–cubic phase transition [
2]. Undoped BaTiO
3 is stable in the hexagonal polymorph at high temperatures (above 1460 °C) and adopts this structure until it melts at 1620 °C [
3]. This hexagonal structure can be stabilized at lower temperatures by a number of B site dopant cations, of comparable size to Ti
4+.
Relying on excellent dielectric, ferroelectric, piezoelectric and optical properties, BaTiO
3 is a well-known electro-ceramic material that is used in the manufacturing of thermistors, dielectric ceramic capacitors [
4,
5,
6] and also applied as photocatalyst and gas sensor material [
7,
8]. These properties of BaTiO
3 are strongly influenced by the existing polymorphs which are linked to the preparation method as well as the dopant. The gas sensor application also depends on the particle size and morphology. In fact, for sensor application, the material needs to adsorb a large quantity of gas on its surface requiring a high surface area and channel-like pore structure. The surface area depends on the particle size and the morphology. The fine spherical morphology offers sufficient surface for gas adsorption, but, must not sinter and cause pore closure. Moreover, the dopant plays a key role in gas sensing (as well as in catalytic NO
x-reduction). Rh dopant contributes in superficial catalytic reactions and can considerably decrease the baseline resistance due to its metallic nature. BaTiO
3 being a dielectric results in a high baseline resistance leading to measurement difficulties in gas sensing. The dopant can also act as a catalyst and, thus, enhance the selectivity of sensing material for a specific gas. During the last decade many efforts have been devoted to the preparation of metal-incorporated or doped BaTiO
3 using different synthesis techniques. These can be divided in two categories: (i) solid-state reaction methods and (ii) wet chemistry methods. The first consists of mixing two solid precursors (e.g., TiO
2 and BaO, or TiO
2 and BaCO
3) and heat treatment of the mixture at high temperatures (≥1000 °C). For instance Ni-doped BaTiO
3 and Mn-doped BaTiO
3 have been prepared by a solid-state reaction method at 1300 °C [
9,
10]. The second method includes hydrothermal, sol-gel, co-precipitation and other techniques. The main advantage of the wet chemistry synthesis techniques is the quasi-atomic dispersion of the dopant as well as Ba and Ti in liquid precursors, which facilitates synthesis of the crystallized powder with nanometer particle sizes usually at low temperatures (<800 °C). Fe-doped BaTiO
3 has been prepared by the hydrothermal route [
11], Co-doped BaTiO
3 by sol-gel route [
12] and Rh-doped BaTiO
3 by hydrothermal and polymerized complex (PC) methods [
13,
14]. The synthesis route plays a very important role on the final properties of the materials [
15,
16,
17] since the morphology, particle size, polymorphs and phase compositions are related directly to the synthesis method and also reflect the desired functional property of the material.
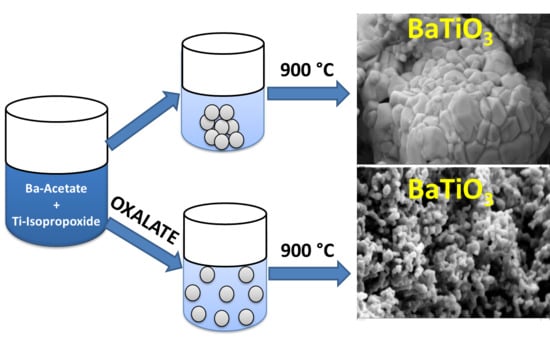
Therefore, this work investigates, to the best of our knowledge for the first time, the effect of the oxalate ligand in co-precipitation route on morphological properties and phase constitution of undoped and Rh-doped BaTiO3. For that, undoped and Rh-doped BaTiO3 is synthesized by co-precipitation method using oxalate as the precipitating agent (achieved for the first time and named hereafter as ‘oxalic route’) and compared with that synthetized by applying a modified solid-state route (hereafter named as ‘classic route’). The effect of these two routes as well as the decomposition temperature on the phase formation, polymorph, morphology, texture and particle size of the as synthesized materials are investigated and the results are compared.
3. Results and Discussions
The phase compositions of all the prepared samples were investigated by XRD and are presented in
Figure 2 and
Figure 3 and
Table 1.
As shown in
Figure 1b the undoped powders synthesized via oxalate route display the presence of only the single phase, BaTiO
3 (according JCPDS 075-0462) (
Figure 2a) at 700 and 900 °C. At 1000°C, in addition to BaTiO
3, as minor phase, orthorhombic BaTi
2O
5 appears. However in the samples prepared by the classic route (
Figure 2b), a mixture of BaCO
3, BaTiO
3, orthorhombic BaTi
2O
5, and TiO
2 rutile is obtained at 700 °C while at 900 °C and 1000 °C BaTiO
3 as major phase and orthorhombic BaTi
2O
5 as minor phase are observed. Additionally, it is noted that single and pure phase of BaTiO
3 can be obtained only with the oxalic route and this forms already at 700 °C. This means that the oxalic route reduces the formation temperature of the pure crystalline phase, barium titanate considerably. This may be due to the presence of oxalate ligand which allows a better distribution and homogeneity of reactant. However the formation of BaTi
2O
5 at 1000 °C indicates that the pure BaTiO
3 obtained through the oxalic route is metastable and decomposes or starts to convert to BaTi
2O
5 phase above 900 °C. We can also observe that as the temperature increases, the XRD peak intensity increases also due certainly to the increase of crystallite size (inset in
Figure 2a).
For the Rh-doped powder prepared via the oxalic route (
Figure 3a), XRD results yielded the presence of the following phases after calcining at 700 °C, 900 °C: BaTiO
3 as the major and BaCO
3 in trace amount. However, at 1000 °C, the powder contained Ba
3RhTi
2O
9 phase in addition to the previous phases obtained at lower temperature. In the presence of Rh doping, we observed the formation of BaCO
3. The same observation has been reported in literature for Cr doped BaTiO
3 [
18]. This can be due to the fact that in those synthesis conditions, a small part of Ba is replaced by a part of Rh (which was not considered during the calculation of the amount of required reactant) and the free barium resulting from this substitution formed easily barium carbonate. This possibility of substitution was also observed in Co-doped BaTiO
3 reported by L. Padilla-Campos et al. [
19].
As the temperature increases, the amount of BaCO
3 decreases due to the decomposition of BaCO
3 and the decrease of the amount of Ba substituted by Rh. No evidence of an Rh phase was encountered at 700 °C and 900 °C. As shown in
Figure 4a, the lattice parameter a increases, implying the cell volume is enlarged due to the larger radius of Rh
3+ (0.67 Å) compared with Ti
4+ (0.60 Å). This increase is higher at 700 °C than at 900 °C (from 4.011 Å to 4.028 Å at 700 °C and from 4.010 Å to 4.015 Å at 900 °C) indicating that the substitution of Ti by Rh is more pronounced at 700 °C. At 1000 °C there was almost no increase of lattice parameter (from 4.009 to 4.010 Å) (
Figure 4a) and there existed another phase which contained rhodium (Ba
3Ti
2RhO
9). It can be suggested that at this temperature, there is no longer substitution of titanium by rhodium. In other words, the Bragg diffraction peaks of powders calcined at this temperature are nearly coincident with the undoped samples. It can be proposed that BaTiO
3 phase incorporating Rh
3+ -ions display a phase change, leading to the formation of a new phase, Ba
3Ti
2RhO
9, due to the diffusion of Rh
3+-ions out of the BaTiO
3 lattice. This might be because of the better phase stability of Ba
3Ti
2RhO
9 even at a temperature of 1000 °C.
Rh-doped powder prepared by the modified classic route (
Figure 3b) contained at 700 °C: BaTiO
3, BaCO
3 and BaTi
2O
5; at 900 °C: BaTiO
3 and BaTi
2O
5 and at 1000 °C: BaTiO
3, BaTi
2O
5 and Ba
3RhTi
2O
9 (see
Table 1). Although at 1000 °C the composition of the doped sample is similar to that obtained by oxalic route, at 700 °C and 900 °C following the classic route leads again to less pure powders due to the reason that was given previously. As shown in
Figure 4b, no evidence for the increase of lattice parameter is observed at 700 °C (from 4.012 Å to 4.013 Å) and there is no decrease of the peak intensity like in oxalic route which means that there was probably no substitution of Ti
4+ by Rh
3+. However at 900 °C, the increase of the lattice parameter is observed (from 4.009 Å to 4.013 Å) with the decrease of peak intensity and at 1000 °C almost no change of lattice parameter is observed (from 4.008 Å to 4.009 Å). The occurrence of the Ti
4+ substitution at lower temperatures by the oxalic route could be explained by the excellent dispersion and homogenization of Rh in the presence of oxalate ligand which in turn allows an easy substitution process. That is not the case with the classic route; therefore the substitution process requires more thermal activation.
In order to confirm the structure of the synthesized materials and to determine the type of BaTiO
3 polymorph obtained, Raman spectroscopy measurements were carried out. Raman scattering spectra are more sensitive to short range ordering structure [
20]. Therefore, while XRD measurement can clarify the average and static symmetries, Raman scattering can clarify the local and dynamic symmetries.
Figure 5 shows the Raman spectra of all the samples. As shown in
Figure 5a–c the Raman spectra of undoped samples prepared by oxalic route at 700, 900 and 1000 °C are different from what obtained by classic route. The samples prepared by co-precipitation yield almost identical peaks to BaTiO
3, with the ones observed at 270, 308, 525 (weak) and 725 (weak) cm
−1 which are attributed respectively to the A1(TO2), E(TO2), A1(TO3) and A1(LO3) of barium titanate modes of the room temperature P4mm phase [
21]. The weakness of the peaks at 725 cm
−1 indicated the presence of a low amount of tetragonal phase suggesting that we have a mixture of cubic (major) and tetragonal phases. However, the samples prepared by the classic route showed additional peaks at 700 °C and 900 °C. The additional peaks are attributed to BaCO
3 (694, 1060 cm
−1), BaTi
2O
5 (880 cm
−1) and TiO
2 (448, 612, 827 cm
−1). At 1000 °C the spectra of samples obtained by the two routes are almost similar indicating the same composition. All these results are in agreement with the XRD analysis. On doping (see
Figure 5d–f), the peak at 725 cm
−1 increases, indicating the increase of tetragonal phase for the samples prepared by the oxalic route. Since Raman scattering can be observed at around 270 and 525 cm
−1 for both tetragonal and cubic symmetries of BaTiO
3 [
22], the tetragonal phase can be determined by observing the relative intensity of the bands at 725 cm
−1. In fact, the band at 725 cm
−1 is related to the highest-wavenumber longitudinal optical mode (LO) of A1 symmetry. Therefore, these bands are absent in the cubic para-electric phase [
23,
24]. Although the observation of this band confirms the presence of the tetragonal phase, this does not exclude a possible cubic phase existence. The relatively intense band at 640 cm
−1 after doping could be assigned to the existence of a hexagonal phase at low temperature usually justified by two Raman bands at 153 and 640 cm
−1 [
22]. In fact at these wavenumbers, the appearance of bands belonging to other barium titanate phases is not expected [
25,
26,
27]. The band at 153 cm
−1 is less intense and, therefore, rarely observed. The hexagonal phase of barium titanate is normally stable at high temperature (1432–1625 °C) and has a TiO
6 octahedra configuration that is significantly different from that of the tetragonal phase. In the tetragonal phase, two TiO
6 octahedra share only one point, while in the hexagonal phase, they share the entire surface [
27]. Yashima et al. [
27] explained the presence of the hexagonal phase at low temperature as the result of a reconstructive phase transition. For our case, the appearance of the hexagonal phase at low temperature can be explained by a local reducing atmosphere during the decomposition of the organic components (oxalate ligand) of the precursor [
28]. In this atmosphere, the reduction of Ti
4+ to Ti
3+ promotes the formation of planar defects so that the stacking sequence in the regions of the defects is equivalent to that of the high-temperature hexagonal barium titanate [
26,
29]. In summary, the presence of dopant seems to promote the formation of tetragonal phase and induce the formation of hexagonal phase. By classic route, the peaks for doped and undoped are almost similar at 700 °C. At 900 °C, some peaks (612, 827 cm
−1) disappear which means that the phase became purer. At 1000 °C some peaks become more intense (725, 640 cm
−1) indicating the increase of the amount of tetragonal and hexagonal phases. We can observe that at 700 °C and 900 °C the peak at 725 cm
−1 is already intense in undoped samples, indicating the presence of tetragonal phase which is not the case with the oxalate route.
The morphology of the particles obtained by the classic and oxalate co-precipitation routes is shown in
Figure 6 and
Figure 7. As it is clear at first glance, the synthesis route and the temperature do have a significant influence on the morphology. The oxalate co-precipitation route leads to homogeneously distributed nano-spherical particles for the undoped BaTiO
3 (
Figure 6b,d) and Rh-doped samples (
Figure 7b,d) on calcining at 700 and 900 °C. However the classic route yields for undoped BaTiO
3 (
Figure 6a,c), large particle blocks already at 700 °C and large faceted particles with a morphology containing stacked and dense blocks formed on recrystallization at 900 °C. These particles then sintered to finer ones in the case of the Rh-doped BaTiO
3 powders (
Figure 7a,c).
The presence of a precipitating agent in the co-precipitation route is certainly responsible for the thermal decomposition driven formation of spherical and homogeneous particles. In fact, the precursor which decomposes thermally contains a mixture of barium titanium rhodium oxalate, resulting in a bonding formation between the metallic Ba2+ or Ti4+ cations and the oxalate ligand (which is not the case for the classic route). The oxalate ligand acts as buffer and prevents the stacking of the particles during thermal treatment. In other word, the presence of a precipitating agent (e.g., in this case oxalate) avoids the stacking of the particles by steric hindrance during thermal decomposition. The thermogravimetric analysis (TGA) measurement (not shown here) of the oxalate route produced powder displays that organic substances leave the system at 700 °C. In fact, the first two steps of a sintering process (particle rearrangement and particle contact through neck formation) occur before the thermal elimination of oxalate ligand is completed. Once the rigid necks are formed, there is no possibility for particles to move together. Thus, the oxalate route produced powder does not yield any volume shrinkage on further temperature increase to 1000 °C. Instead, the next stage of the sintering process, i.e., grain growth takes place. Thus, in conclusion, the oxalate processed material experiences retardation in the sintering process. Moreover, above the oxalate decomposition temperature (650–700 °C), the sintering process may proceed with the grain growth stage but owing to the space left behind from the decomposing and vaporizing organic part, the growth rate of these particles is low. This is because solid/solid interaction occurs only at the neck points yielding extension of particles through the transfer of material to the neck points. In the case of the classic route, where there was no organic ligand addition, the processes such as heavy particle rearrangement and particle pulling-together result in contact all along the particle grain boundaries. Thus, a strong shrinkage takes place followed by a solid state sintering process and grain growth. This is confirmed by different SEM pictures of the classic route where it can be observed that the particles are highly sintered together and are grown in comparison to those in the oxalate route produced material.
It is also notable that Rh-doping results in a decrease in the size of the individual particles or of the particles in a block with the oxalic route or with the classic route, respectively. This observation is in accordance with XRD where we observed the decrease of peak intensities with the doping. On further heat-treatment of the powders synthesized by the classic route at 1000 °C, the large dense blocks, built of rhombohedral crystallites are formed (
Figure 6e and
Figure 7e). In turn, the oxalate co-precipitation route yields only larger aggregates of spherical nanoparticles (
Figure 6f and
Figure 7f). At this temperature, it appears that the Rh-doping at both routes results in an enhancement of the agglomeration process. It is observed that the increase of the heat-treatment temperature leads to an increase in particle mobility resulting in particle growth and this is in agreement with XRD results.
Energy-dispersive X-ray spectroscopy (EDX) is an analytical technique used for elemental analysis. The compositions of all 4 samples (BT-OX-700, BT-CL-700, BTR1-OX-700 and BTR1-OX-700) taken as representative of all the samples are presented in
Table 2. In order to check the homogeneity of the samples, the measurements were done in three different areas. As can be observed, the samples prepared by the oxalic route showed almost the same composition everywhere with Ti/Ba ratio equal roughly to 1, indicating the good homogeneity and this is not the case with the samples prepared by classic route (e.g., sample BT-CL-700). For the undoped samples prepared by the oxalic route, the mean atomic percentages obtained from EDX quantification analysis were 21.34% of Ti (cf 20% expected), 18.85% of Ba (cf 20% expected) and 59.80% for O (cf 60% expected) which corresponded to BaTiO
3 as revealed by XRD and Raman. After doping, the percentages of Ti and Ba dropped to low values (although the ratio between Ti/Ba did not change greatly), due certainly to the presence of additional compounds which increase the total number of atom. This is confirmed by the detection of BaCO
3 revealed by XRD after Rh doping.
The specific surface areas of the synthesized samples at 900 °C and 1000 °C taken as representative of all the samples obtained by BET measurements are presented in
Table 3. These results indicate that the specific surface area of the powders synthesized by the oxalic route and calcined at 900 °C is a factor of four greater than that obtained with the powder synthesized by classic route, while this relation is reduced roughly to a factor of two after calcining the powders at 1000 °C. This difference in the surface area could be related to the morphological distribution of the particles. In fact as observed by SEM, the spherical nanoparticles obtained by the oxalic route may offer more freely available surfaces for the gas adsorption such as N
2 during BET measurements while the stacked and dense blocks obtained with the classic route do not allow the inter-diffusion of nitrogen. It should also be mentioned that the specific surface areas of doped and undoped powders calcined at 900 °C differ, indicating an increase with the Rh-doping. This increase is in agreement with the SEM and XRD results displaying a decrease in the particle and block sizes for the Rh-doped powders synthesized with the oxalic and classic routes, respectively. The reduction of specific surface area observed with the powders calcined at 1000 °C can be explained relying on the microstructural observations by means of SEM that the particle agglomeration occurs as the heat-treatment temperature increases, leading to the reduction of surface area that is available for gas adsorption. This agglomeration is more pronounced at the powders synthesized by means of the classic route where the formation of dense blocks is obvious at the SEM micrographs. In the case of the oxalic route, the presence of precipitation agent might have limited this agglomeration, since the micrographs display a more open spaced microstructure. It should be noticed that there is a reduction of specific surface area with the doping at 1000 °C for the oxalic route-synthesized powder, due certainly to the increase of agglomeration with doping as observed on SEM.
In summary, Rh-doped BaTiO3 powders synthesized by the oxalic route offers the following features: (i) high phase purity, (ii) high surface area, (iii) sintering and growth resistant nano-sized spherical particles, (iv) thermal phase stability of BaTiO3 up to 1000 °C. These features can be effectively used to enhance the functional properties of barium titanate such as gas sensor which will be investigated in the future.