Exogenous Production of Silver Nanoparticles by Tephrosia apollinea Living Plants under Drought Stress and Their Antimicrobial Activities
Abstract
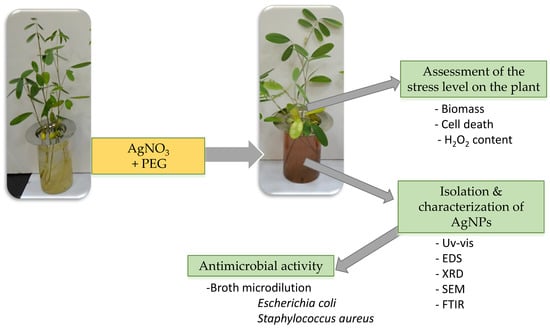
:1. Introduction
2. Materials and Methods
2.1. Seed Germination
2.2. Preparation of Treatment Solutions and Plant Harvest
2.3. Evan’s Blue Staining
2.4. Measurement of H2O2 Content
2.5. Nanoparticle Characterization
2.6. Antimicrobial Broth Microdilution Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of AgNO3 and PEG on Plant Phenotype and Biomass
3.2. AgNO3 and PEG Exhibited Increase in T. apollinea EC
3.3. AgNO3 and PEG Stresses Reduced Viability of T. apollinea Root Cells
3.4. AgNO3 and PEG Exposure Caused Oxidative Stress in T. apollinea Roots
3.5. Detection of Exogenous AgNPs Synthesised by T. apollinea Plants
3.6. XRD Analysis of the Synthesized Nanoparticles
3.7. Encapsulation of AgNPs and T. apollinea Phytochemicals
3.8. Phytosenthesized AgNP Produced by T. apollinea Exhibited Antimicrobial Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasad, R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanopart. 2014, 2014, 963961. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. EDP Sci. 2017, 129, 02013. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, J.S.; Singh, D.P. Biogenic synthesis and spatial distribution of silver nanoparticles in the legume mungbean plant (Vigna radiata L.). Plant Physiol. Biochem. 2017, 110, 158–166. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Raju, D.; Paneliya, N.; Mehta, U. Extracellular synthesis of silver nanoparticles using living peanut seedling. Appl. Nanosci. 2014, 4, 875–879. [Google Scholar] [CrossRef]
- Marchiol, L. Synthesis of metal nanoparticles in living plants. Ital. J. Agron. 2012, 7, e37. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Raju, D.; Mehta, U.J.; Ahmad, A. Phytosynthesis of intracellular and extracellular gold nanoparticles by living peanut plant (Arachis hypogaea L.). Biotechnol. Appl. Biochem. 2012, 59, 471–478. [Google Scholar] [CrossRef]
- Vijayalakshmi, M.; Akilandeswari, K.; Kavitha, K.; Gokila, S.; Vinothkumar, R. Green synthesis and antibacterial activity of silver nanoparticle using Cressa cretica plant extract. Chem. Lett. 2018, 1, 12–18. [Google Scholar]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Al-Nuairi, A.G.; Mosa, K.A.; Mohammad, M.G.; El-Keblawy, A.; Soliman, S.; Alawadhi, H. Biosynthesis, Characterization, and Evaluation of the Cytotoxic Effects of Biologically Synthesized Silver Nanoparticles from Cyperus conglomeratus Root Extracts on Breast Cancer Cell Line MCF-7. Biol. Trace Elem. Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef]
- Rajaram, K.; Aiswarya, D.C.; Sureshkumar, P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater. Lett. 2015, 138, 251–254. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Benelli, G.; Kumar, G.; Pugazhendhi, A.; Kim, D.S.; Shin, H.S. Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J. Clust. Sci. 2017, 28, 1709–1727. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, Characterization, and Evaluation of the Antibacterial Activity of Allophylus serratus Leaf and Leaf Derived Callus Extracts Mediated Silver Nanoparticles. J. Nanomater. 2017, 2017, 4213275. [Google Scholar] [CrossRef]
- Jongbloed, M. The Comprehensive Guide to the Wild Flowers of the United Arab Emirates; E.R.W.D.A.: Abu Dhabi, UAE, 2003. [Google Scholar]
- Ghazanfar, S.A.; Al-Sabahi, A.M.A. Medicinal Plants of Northern and Central Oman (Arabia). Econ. Bot. 1993, 47, 89–98. [Google Scholar] [CrossRef]
- Cheruth, A.J.; Al Baloushi, S.A.M.; Karthishwaran, K.; Maqsood, S.; Kurup, S.S.; Sakkir, S. Medicinally active principles analysis of Tephrosia apollinea (Delile) DC. growing in the UAE. BMC Res. Notes 2017, 10, 61. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 164–172. [Google Scholar] [CrossRef]
- Jisha, E.R.; Balamurugan, G.; Edison, N.; Selvakumar, P.; Rathiga, R. Biogenic synthesis of Gold Nanoparticles using leaf extract of Tephrosia purpurea and study of their antibacterial effect. Int. J. Pharm Tech Res. 2012, 4, 1323–1331. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Mauro, N.; Schillaci, D.; Varvarà, P.; Cusimano, M.G.; Geraci, D.M.; Giuffrè, M.; Cavallaro, G.; Maida, C.M.; Giammona, G. Branched High Molecular Weight Glycopolypeptide With Broad-Spectrum Antimicrobial Activity for the Treatment of Biofilm Related Infections. ACS Appl. Mater. Interfaces 2018, 10, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, I.M.; Hameed, M.K.; Almehdi, A.M.; Arooj, M.; Workie, B.; Sahle-Demessie, E.; Han, C.; Mohamed, A.A. Green and Cytocompatible Carboxyl Modified Gold-Lysozyme Nanoantibacterial for Combating Multidrug-Resistant Superbugs. Biomater. Sci. 2019, 7, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- Erjaee, H.; Rajaian, H.; Nazifi, S. Synthesis and characterization of novel silver nanoparticles using Chamaemelum nobile extract for antibacterial application. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 25004. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 30 November 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 0.7.8. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 November 2019).
- Pardha-Saradhi, P.; Yamal, G.; Peddisetty, T.; Sharmila, P.; Nagar, S.; Singh, J.; Nagarajan, R.; Rao, K.S. Reducing Strength Prevailing at Root Surface of Plants Promotes Reduction of Ag+ and Generation of Ag0/Ag2O Nanoparticles Exogenously in Aqueous Phase. PLoS ONE 2014, 9, e106715. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharreddy, P.; Rani, P.U.; Sreedhar, B. Qualitative assessment of silver and gold nanoparticle synthesis in various plants: A photobiological approach. J. Nanopart. Res. 2010, 12, 1711–1721. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Shitole, S.M.; Dhumal, K.N. Effect of water stress by polyethylene glycol 6000 and sodium chloride on seed germination and seedling growth of Cassia angustifolia. Int. J. Pharm. Sci. Res. 2012, 3, 528. [Google Scholar]
- Slama, I.; Ghnaya, T.; Hessini, K.; Messedi, D.; Savouré, A.; Abdelly, C. Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ. Exp. Bot. 2007, 61, 10–17. [Google Scholar] [CrossRef]
- Jiang, H.; Li, M.; Chang, F.; Li, W.; Yin, L. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Danwanichakul, P.; Suwatthanarak, T.; Suwanvisith, C.; Danwanichakul, D. The Role of Ammonia in Synthesis of Silver Nanoparticles in Skim Natural Rubber Latex. J. Nanosci. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Suwatthanarak, T.; Than-ardna, B.; Danwanichakul, P.; Danwanichakul, D. Synthesis of silver nanoparticles in skim natural rubber latex at room temperature. Mater. Lett. 2016, 168, 31–35. [Google Scholar] [CrossRef]
- Tien, D.; Tseng, K.; Liao, C.; Huang, J.; Tsung, T. Discovery of ionic silver in silver nanoparticle suspension fabricated by arc discharge method. J. Alloys Compd. 2008, 463, 408–411. [Google Scholar] [CrossRef]
- Qiu, C.; Xiao, X.; Liu, R. Biomimetic synthesis of spherical nano-hydroxyapatite in the presence of polyethylene glycol. Ceram. Int. 2008, 34, 1747–1751. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Panda, B.B.; Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitr. 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and Genotoxic Effects of Copper Nanoparticles in Coriander (Coriandrum sativum -Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Zlatev, Z.; Lidon, F.; Ramalho, J.; Yordanov, I. Comparison of resistance to drought of three bean cultivars. Biol. Plant 2006, 50, 389–394. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relationsand photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper nanoparticles induced genotoxicty, oxidative stress, and changes in Superoxide Dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [Green Version]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamal, G.; Sharmila, P.; Rao, K.S.; Pardha-Saradhi, P. Inbuilt Potential of YEM Medium and Its Constituents to Generate Ag/Ag2O Nanoparticles. PLoS ONE 2013, 8, e61750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci. Rep. 2018, 8, 8925. [Google Scholar] [CrossRef]
- Ganesan, V.; Aruna Devi, J.; Astalakshmi, A.; Nima, P.; Thangaraja, A. Eco-friendly synthesis of silver nanoparticles using a sea weed, Kappaphycus alvarezii (Doty) Doty ex PC Silva. Int. J. Eng. Adv. Technol. 2013, 2, 559–563. [Google Scholar]
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J.C.; Panwar, J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: A mechanism perspective. Nanoscale 2011, 3, 635–641. [Google Scholar] [CrossRef]
- Salehi, S.; Shandiz, S.A.S.; Ghanbar, F.; Darvish, M.R.; Ardestani, M.S.; Mirzaie, A.; Jafari, M. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int. J. Nanomed. 2016, 11, 1835–1846. [Google Scholar]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Kokati Venkata, B.R. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Pawar, O.; Deshpande, N.; Dagade, S.; Waghmode, S.; Nigam Joshi, P. Green synthesis of silver nanoparticles from purple acid phosphatase apoenzyme isolated from a new source Limonia acidissima. J. Exp. Nanosci. 2016, 11, 28–37. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.V.; Nachane, R.P.; Balasubramanya, R.H. Silver−Protein (Core−Shell) Nanoparticle Production Using Spent Mushroom Substrate. Langmuir ACS J. Surf. Colloids 2007, 23, 7113–7117. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Salayová, A. Green-Synthesized Silver Nanoparticles and Their Potential for Antibacterial Applications. In Bacterial Pathogenesis and Antibacterial Control, 1st ed.; Kırmusaoğlu, S., Ed.; IntechOpen: London, UK, 2018; pp. 73–94. [Google Scholar]
- Yun’an Qing, L.C.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Kassas, H.Y.; Attia, A.A. Bactericidal Application and Cytotoxic Activity of Biosynthesized Silver Nanoparticles with an Extract of the Red Seaweed Pterocladiella capillacea on the HepG 2 Cell Line. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 1299–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Treatment | Day | E. coli | S. aureus | ||||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||||
| 1 mM AgNO3 | 3 | 125 | 1000 | 31.25 | >1000 | ||
| 6 | 125 | 500 | 62.5 | >1000 | |||
| 1 mM AgNO3+ | −0.1 | Osmotic Potential (MPa) PEG | 3 | 62.5 | 250 | 31.25 | >1000 |
| 6 | 125 | 250 | 62.5 | >1000 | |||
| −0.2 | 3 | 31.25 | 125 | 15.625 | >1000 | ||
| 6 | 62.5 | 500 | 62.5 | >1000 | |||
| −0.4 | 3 | 31.25 | 125 | 15.625 | >1000 | ||
| 6 | 31.25 | 125 | 15.625 | >1000 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.A.; Mosa, K.A.; El-Keblawy, A.; Alawadhi, H. Exogenous Production of Silver Nanoparticles by Tephrosia apollinea Living Plants under Drought Stress and Their Antimicrobial Activities. Nanomaterials 2019, 9, 1716. https://doi.org/10.3390/nano9121716
Ali MA, Mosa KA, El-Keblawy A, Alawadhi H. Exogenous Production of Silver Nanoparticles by Tephrosia apollinea Living Plants under Drought Stress and Their Antimicrobial Activities. Nanomaterials. 2019; 9(12):1716. https://doi.org/10.3390/nano9121716
Chicago/Turabian StyleAli, Muna A., Kareem A. Mosa, Ali El-Keblawy, and Hussain Alawadhi. 2019. "Exogenous Production of Silver Nanoparticles by Tephrosia apollinea Living Plants under Drought Stress and Their Antimicrobial Activities" Nanomaterials 9, no. 12: 1716. https://doi.org/10.3390/nano9121716
APA StyleAli, M. A., Mosa, K. A., El-Keblawy, A., & Alawadhi, H. (2019). Exogenous Production of Silver Nanoparticles by Tephrosia apollinea Living Plants under Drought Stress and Their Antimicrobial Activities. Nanomaterials, 9(12), 1716. https://doi.org/10.3390/nano9121716