A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles
Abstract
1. Introduction
2. Classification of Nanomaterials
2.1. Inorganic Nanoparticles
2.2. Metal Oxide Nanoparticles
2.3. Organic Nanoparticles
2.4. Carbon-based Nanoparticle
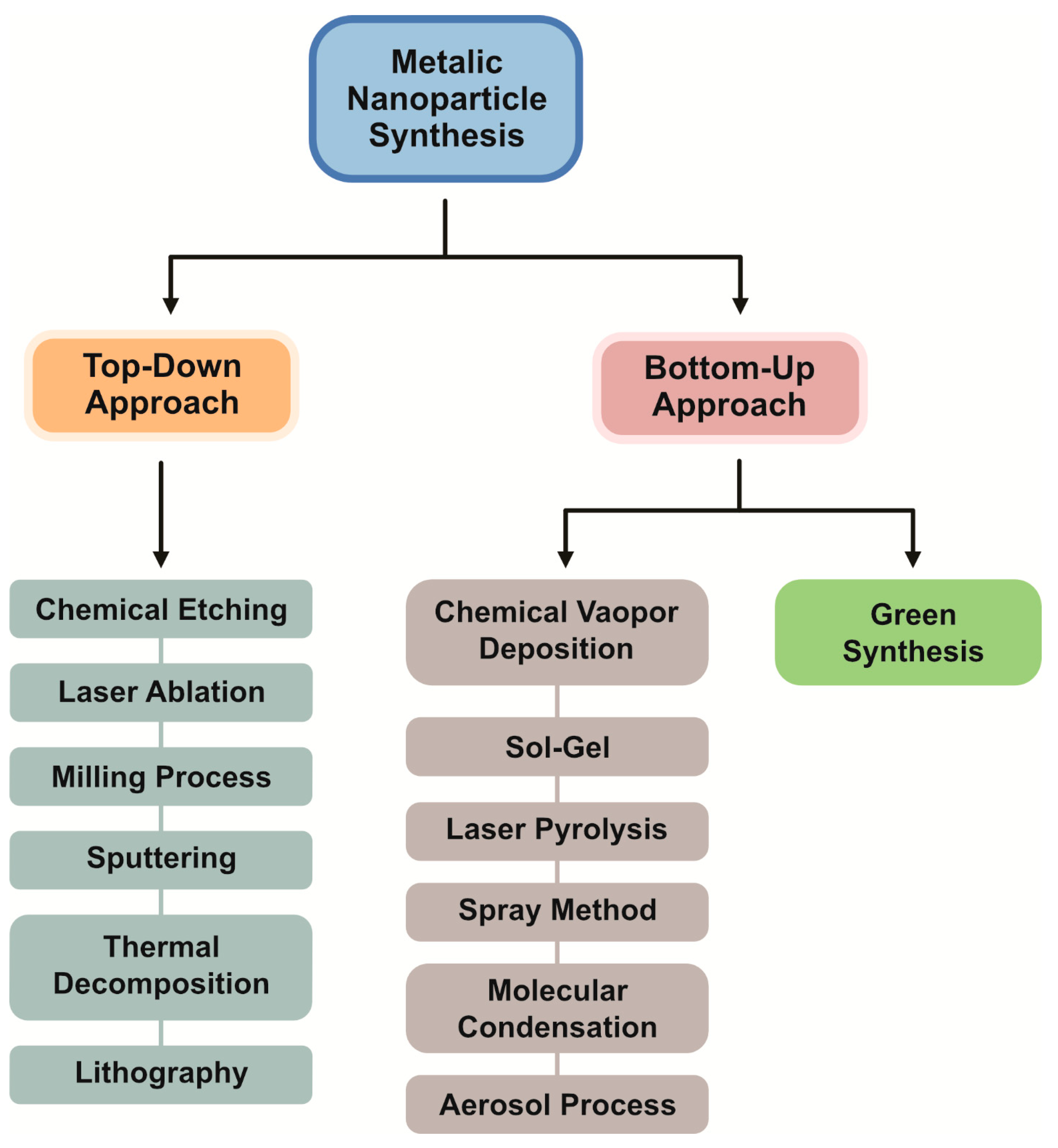
3. Methods for Synthesis of PtNPs
3.1. Bottom-Up Approaches
- (1)
- Top-down approaches
- (2)
- Bottom-up approaches
3.2. Physical Methods
3.3. Chemical Methods
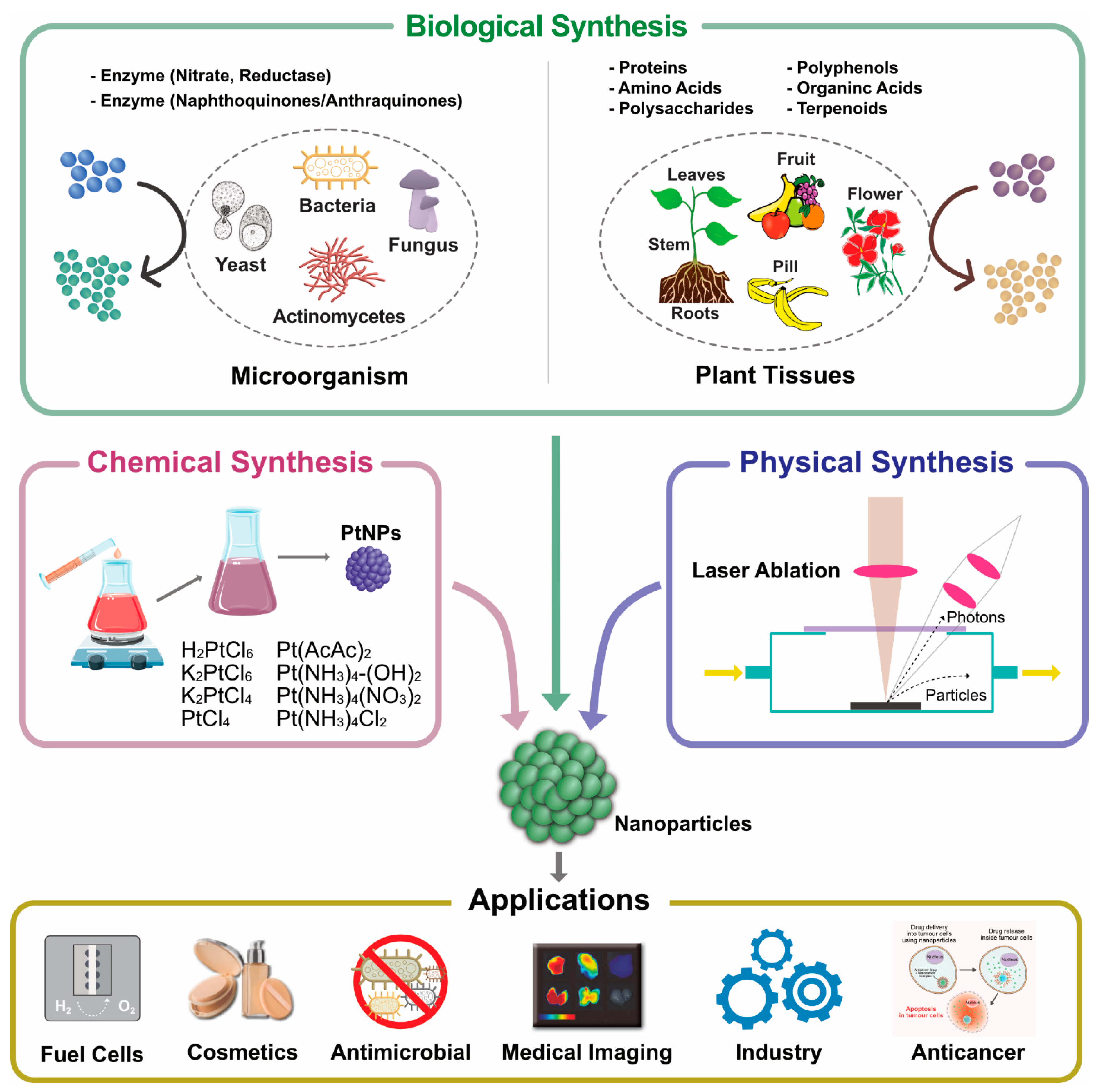
3.4. Biological Synthesis of PtNPs
3.5. Synthesis of Platinum Nanoparticle Using Bacteria
3.6. Synthesis of Platinum Nanoparticles Using Fungi
3.7. Green Synthesis of Platinum Nanoparticles Using Plants
3.8. Synthesis of Platinum Nanoparticles Using Purified Plant Compounds
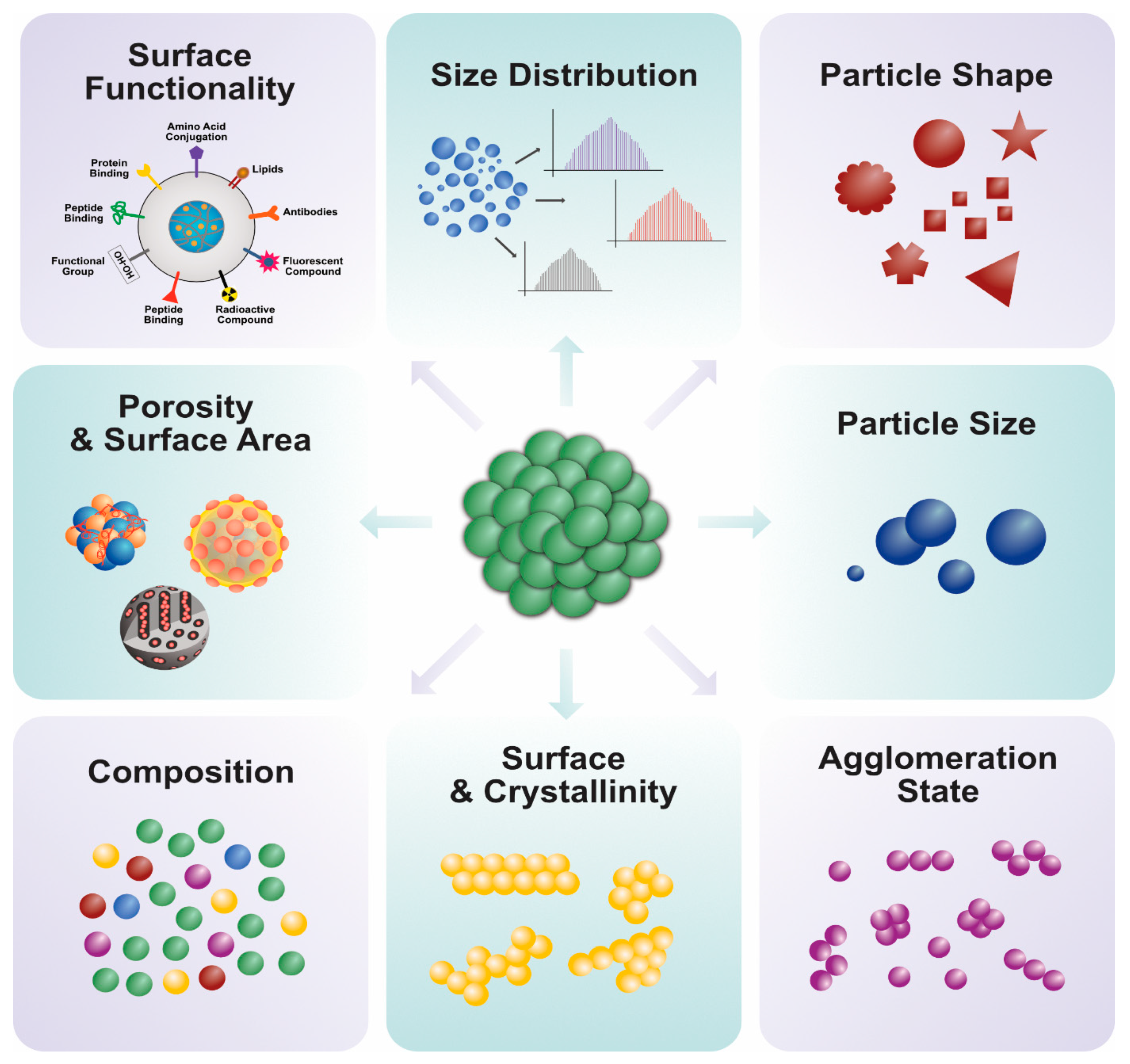
4. Characterization of PtNPs Using Various Analytical Techniques
4.1. UV–Visible Spectroscopy
4.2. Fourier Transform Infrared Spectroscopy
4.3. Nanoscale Infrared Spectroscopy
4.4. Dynamic Light Scattering
4.5. Electrophoretic Light Scattering (ELS)
4.6. XRD
4.7. Scanning Electron Microscopy (SEM)
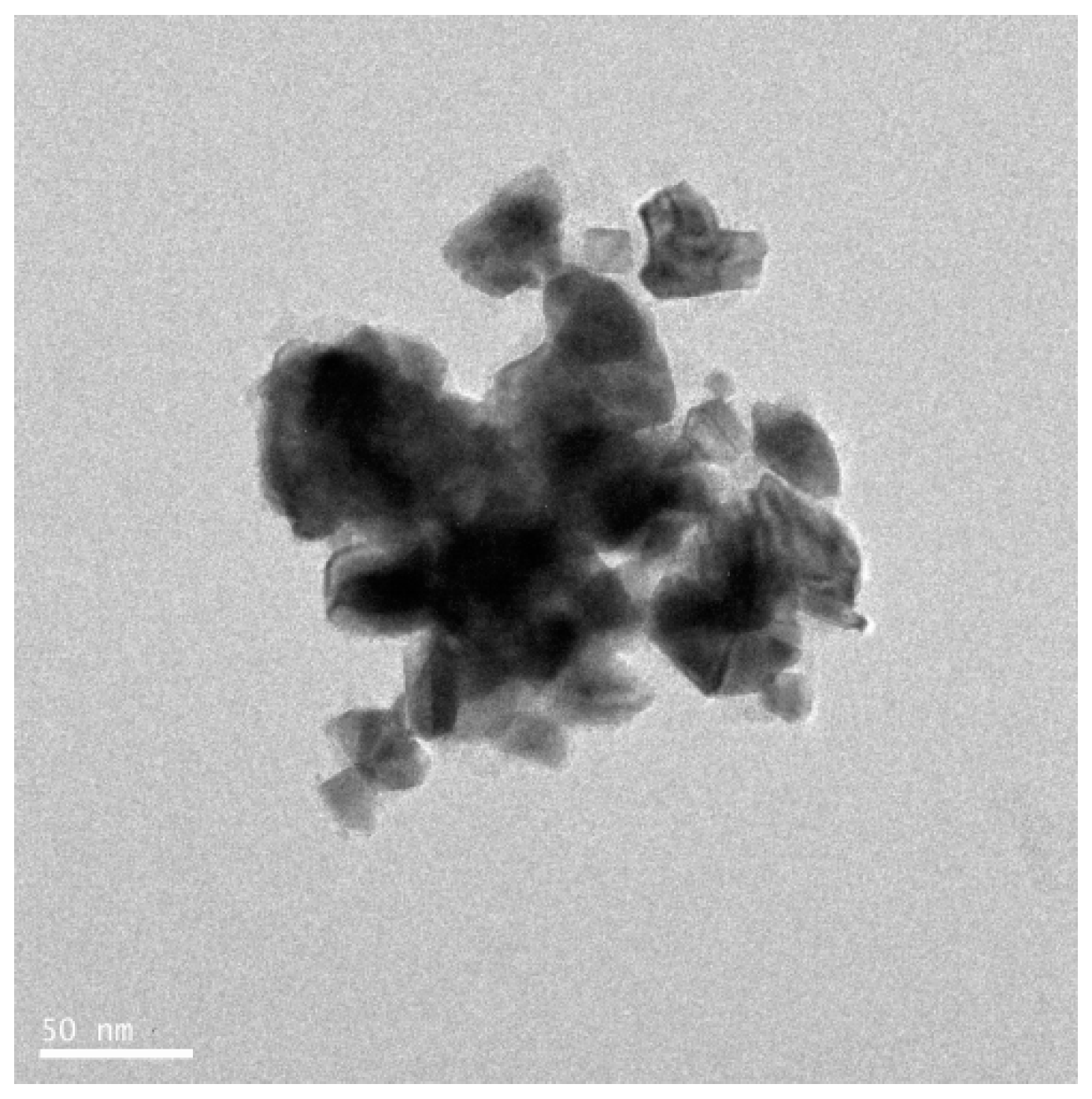
4.8. TEM
4.9. AFM
4.10. EXAFS
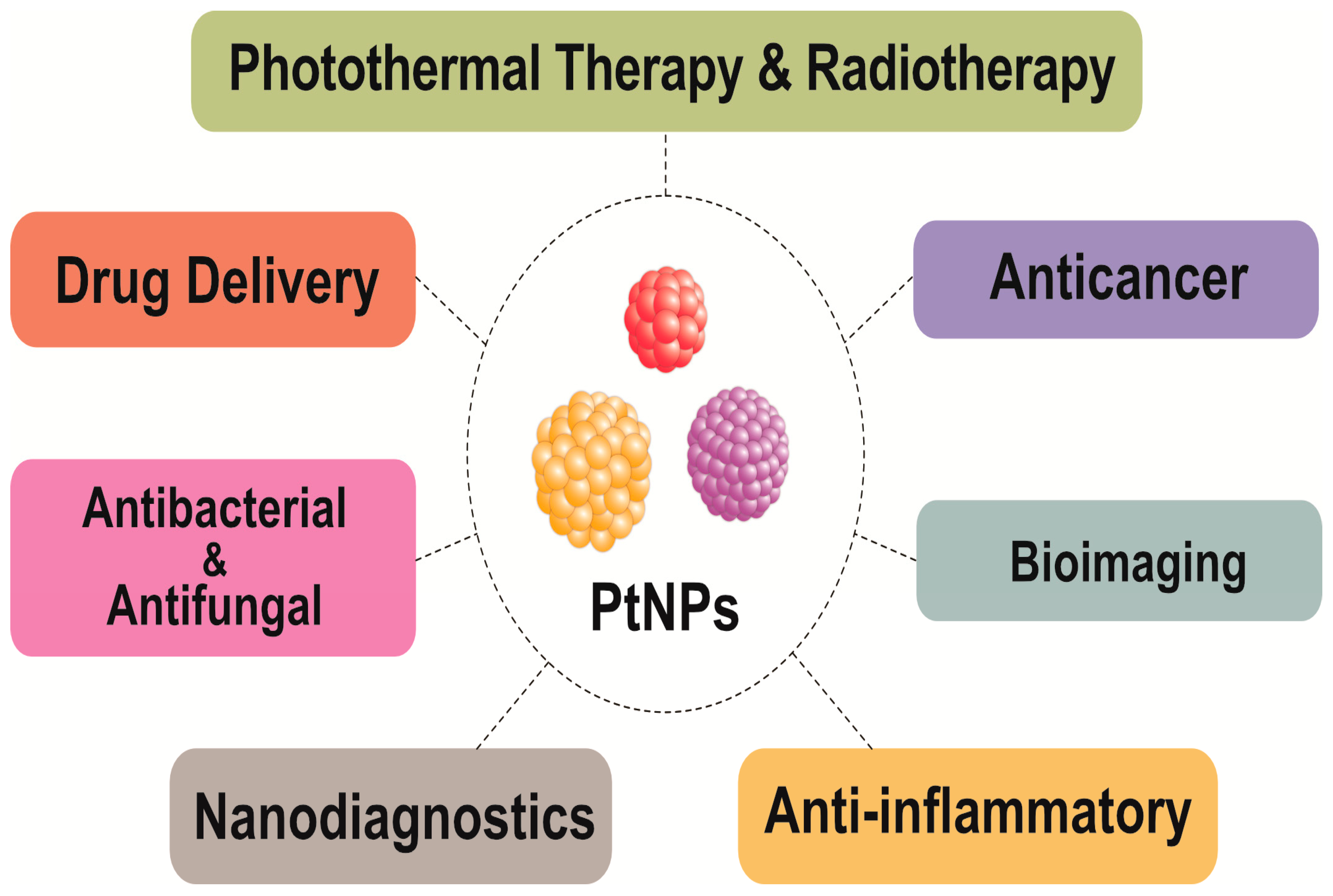
5. Multifarious Applications of PtNPs
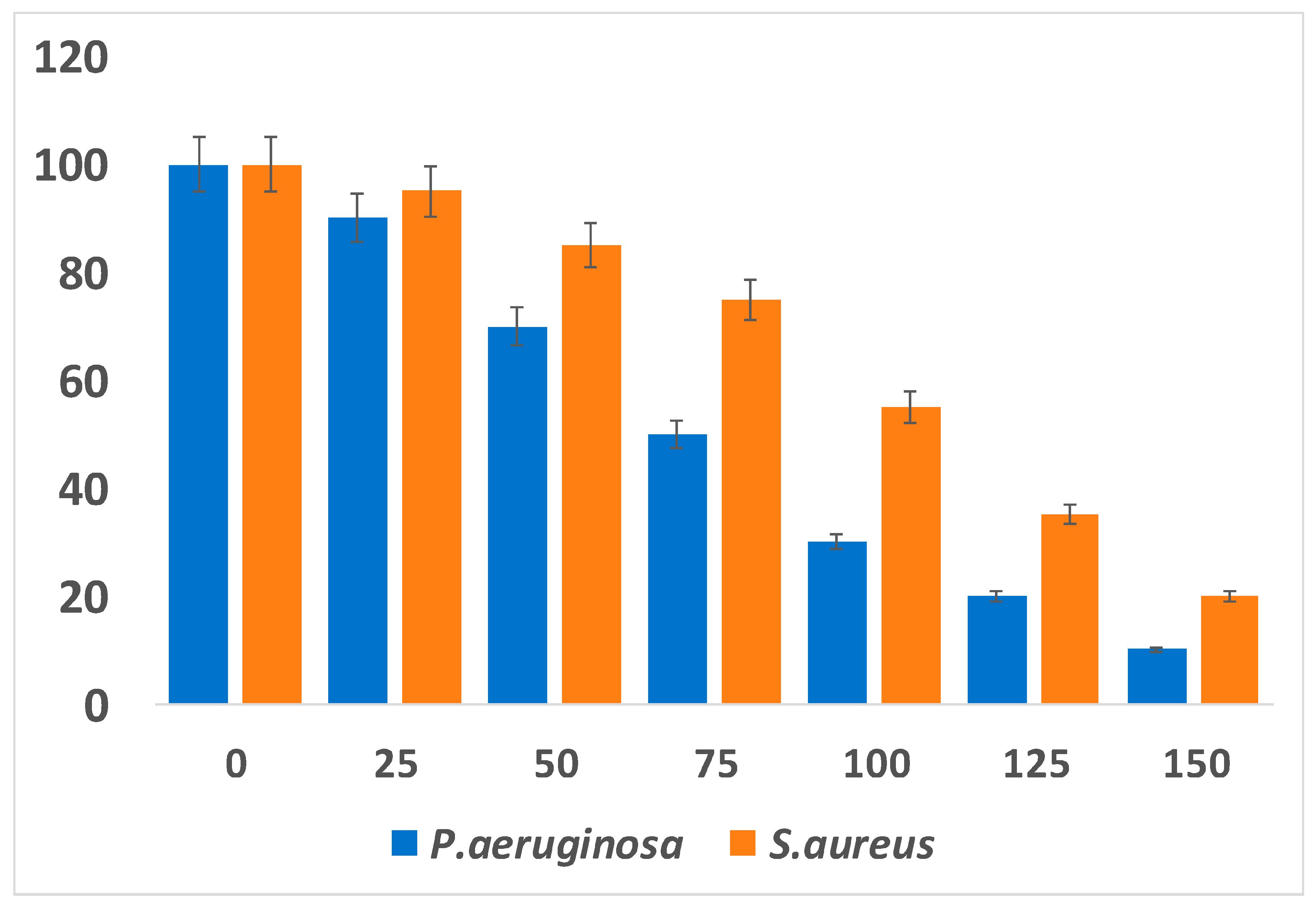
6. Antibacterial Activity of Platinum Nanoparticle
7. Antifungal Activity of Platinum Nanoparticles
7.1. Anticancer Activity of Platinum
7.2. Cytotoxicity of PtNPs in Cancer and Non-Cancer Cells
8. In Vivo Toxicity of PtNPs
8.1. Use of PtNPs in Combination Therapy
8.2. Biomedical Applications of PtNPs
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Name of the Organism | Parts Uses | Size (nm) | Shape | Ref. |
|---|---|---|---|---|
| Bacteria | ||||
| Desulfovibrio vulgaris | Periplasmic space/cellular surface | [71] | ||
| Acinetobacter calcoaceticus | Intracellular | 2–3.5 | Cuboidal | [72] |
| Fungi | ||||
| F. oxysporum | Extracellular | 10–50 | triangle, hexagons, square, rectangles | [74] |
| Neurospora Crassa | Intracellular | 2–3, 4–35, 7–76 and 20–110 | Quasi spherical, single crystalline and round nano aggregates | [80] |
| Plants | ||||
| Doipyros kaki | Leaf | 2–12 | [82] | |
| Fumariae herba | Whole | 30 | Hexagonal and pentagonal | [83] |
| Anacardium occidentale | Leaf | 100 | Irregular rod | [84] |
| Azadirachta indica | Leaf | 5–50 | Small and large sphere | [85] |
| Piper betle L. | Leaf | 2–0.4 | Spherical | [86] |
| Ocimun sanctum | Leaf | 23 | Irregular | [87] |
| Phoenix dactylifera | Fruit | 1.3–2.6 | Spherical | [91] |
| Lantana camara | Leaf | 35 | Spherical | [93] |
| Prunus x yedoensis | Gum | 10–20 | Circular | [94] |
| Camellia sinensis | Leaf | 30–60 | Flower | [95] |
| Antigonon leptopus | Whole plant | 5–190 | Spherical | [96] |
| B. prionitis | Leaf | 1–2 | Monodispersed | [97] |
| Punica granatum | Peel | 16–23 | Spherical | [98] |
| Pomegranate | 20.12 | Spherical | [100] | |
| Algae | ||||
| Padina gymnospora | 25 | Octahedral | [172] | |
| Bacteria | ||||
| Saccharomy cesboulardii | Intracellular | 80–150 | [193] | |
| Plants | ||||
| Eichhornia crassipes | Leaf | 3.74 | Spherical | [255] |
| Quercus glauca | Leaf | 5–15 | Spherical | [256] |
| Bacopa Monnieri | Leaf | 5–20 | Spherical | [257] |
| Cochlospermum gossypium | Tree-Gum | 2.4 | Spherical | [258] |
| Algae | ||||
| Plectonema boryanum UTEX 485 | Cell extract | <300 | Spherical | [259] |
| Bacteria | ||||
| Calothrixv cyanobacteria | Intracellular and extracellilar | 3.2 | [260] | |
| Plants | ||||
| Pinus resinosa | Bark | 6–8 | Irregular | [261] |
| Gloriosa superb | Tuber | 0.83–3 | Spherical | [262] |
| Terminalia chebula | Fruit | <4 | Spherical | [263] |
| Cacumen platycladi | Whole plant | 2.4-0.8 | spherical | [264] |
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Nie, Z.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Sawadaishi, T. Bottom-up strategy of materials fabrication: A new trend in nanotechnology of soft materials. Curr. Opin. Colloid Interface Sci. 2001, 6, 11–16. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, L.; Huang, C.; Huang, Y.; Jia, N. Albumin-mediated platinum nanocrystals for in vivo enhanced computed tomography imaging. J. Mater. Chem. B 2017, 5, 3498–3510. [Google Scholar] [CrossRef]
- Doherty, R.E.; Sazanovich, I.V.; McKenzie, L.K.; Stasheuski, A.S.; Coyle, R.; Baggaley, E.; Bottomley, S.; Weinstein, J.A.; Bryant, H.E. Photodynamic killing of cancer cells by a Platinum(II) complex with cyclometallating ligand. Sci. Rep. 2016, 6, 22668. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Fan, J.; Tan, Y.; Zheng, N. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721. [Google Scholar] [CrossRef]
- Tsung, C.-K.; Kuhn, J.N.; Huang, W.; Aliaga, C.; Hung, L.-I.; Somorjai, G.A.; Yang, P. Sub-10 nm platinum nanocrystals with size and shape control: Catalytic study for ethylene and pyrrole hydrogenation. J. Am. Chem. Soc. 2009, 131, 5816–5822. [Google Scholar] [CrossRef]
- Miyake, M.; Miyabayashi, K. Shape and size-controlled Pt nanocrystals as novel catalysts. Catal. Surv. Asia 2012, 16, 1–13. [Google Scholar] [CrossRef]
- Schmidt, E.; Vargas, A.; Mallat, T.; Baiker, A. Shape-selective enantioselective hydrogenation on Pt nanoparticles. J. Am. Chem. Soc. 2009, 131, 12358–12367. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Somorjai, G.A. Nanoscale advances in catalysis and energy applications. Nano Lett. 2010, 10, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.T.; Ahmed, E.H.; Christensen, C.H.; Fehrmann, R.; Riisager, A. Hydrodeoxygenation of waste fat for diesel production: Study on model feed with Pt/alumina catalyst. Fuel 2011, 90, 3433–3438. [Google Scholar] [CrossRef]
- Lee, I.; Delbecq, F.; Morales, R.; Albiter, M.A.; Zaera, F. Tuning selectivity in catalysis by controlling particle shape. Nat. Mater. 2009, 8, 132–138. [Google Scholar] [CrossRef]
- Kliewer, C.J.; Somorjai, G.A. Structure effects on Pyridine hydrogenation over Pt (111) and Pt (100) studied with sum frequency generation vibrational spectroscopy. Catal. Lett. 2010, 137, 118–122. [Google Scholar] [CrossRef]
- Kasem, K.K. Role of Platinum in Photoelectrochemical Studies Related to Solar Energy Harvesting. Platin. Met. Rev. 2012, 56, 221–228. [Google Scholar] [CrossRef]
- Kumakiri, I.; Diplas, S.; Simon, C.; Nowak, P. Photocatalytic membrane contactors for water treatment. Ind. Eng. Chem. Res. 2011, 50, 6000–6008. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, K.; Peng, S.; Lu, G.; Li, J.S. Photocatalytic hydrogen generation in the presence of ethanolamines over Pt/ZnIn2S4 under visible light irradiation. Mol. Catal. A Chem. 2012, 363, 354–361. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Honda, A.; Zhao, Q.L.; Makino, T.; Abe, R.; Matsui, K.; Shimizu, H.; Miyamoto, Y.; Kondo, T.; Shimizu, T. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 2010, 19, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, H.; Haruki, R.; Yamada, K.; Böttcher, C.; Komatsu, T. Hemoglobin-albumin cluster incorporating a Pt nanoparticle: Artificial O2 carrier with antioxidant activities. PLoS ONE 2014, 9, e110541. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Porcel, E.; Remita, H.; Marco, S.; Réfrégiers, M.; Dutertre, M.; Confalonieri, F.; Lacombe, S. Platinum nanoparticles: An exquisite tool to overcome radioresistance. Cancer Nano. 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Pokropivny, V.V.; Skorokhod, V.V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. World J. Appl. Sci. 2008, 3, 417–433. [Google Scholar]
- Pease, R.F.; Chou, S.Y. Lithography and Other Patterning Techniques for Future Electronics. Proc. IEEE 2008, 96, 248–270. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.; Verma, N.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Nichols, W.T.; Sasaki, T.; Koshizaki, N. Laser ablation of a platinum target in water. III. Laser-induced reactions. J. Appl. Phys. 2006, 100, 114911. [Google Scholar] [CrossRef]
- Mafune, F.; Kondow, T. Selective laser fabrication of small nanoparticles and nano-networks in solution by irradiation of UV pulsed laser onto platinum nanoparticles. Chem. Phys. Lett. 2004, 383, 343–347. [Google Scholar] [CrossRef]
- Park, D.K.; Lee, S.J.; Lee, J.; Choi, M.Y.; Han, S.W. Effect of polymeric stabilizers on the catalytic activity of Pt nanoparticles synthesized by laser ablation. Chem. Phys. Lett. 2010, 484, 254–257. [Google Scholar] [CrossRef]
- Cueto, M.; Sanz, M.; Oujja, M.; Gamez, F.; Haya, M.B.; Castillejo, M. Platinum Nanoparticles Prepared by Laser Ablation in Aqueous Solutions: Fabrication and Application to Laser Desorption Ionization. J. Phys. Chem. C. 2011, 115, 22217–22224. [Google Scholar] [CrossRef]
- Maicu, M.; Schmittgens, R.; Hecker, D.; Glob, D.; Frach, P.; Gerlach, G. Synthesis and deposition of metal nanoparticles by gas condensation process. J. Vac. Sci. Technol. A Vac. Surf. Films 2014, 32, 02B113. [Google Scholar] [CrossRef]
- Stepanov, A.L.; Golubev, A.N.; Nikitin, S.I.; Osin, Y.N. A Review on the fabrication and properties of platinum nanoparticles. Rev. Adv. Mater. Sci. 2014, 38, 160–175. [Google Scholar]
- Adams, D. Inorganic Solids: An Introduction to Concepts in Solid State Structural Chemistry; John Wiley & Son: London, UK; New York, NY, USA; Sydney, Australia; Toronto, ON, Canada, 1974. [Google Scholar]
- Peng, Z.A.; Peng, X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
- Qu, L.; Peng, Z.A.; Peng, X. Alternative routes toward high quality CdSe nanocrystals. Nano Lett. 2001, 1, 333–337. [Google Scholar] [CrossRef]
- Yamada, M.; Foote, M.; Prow, T.W. Therapeutic gold, silver, and platinum nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 428–445. [Google Scholar] [CrossRef]
- Adlim, M.; Abu Bakar, M.; Liew, K.Y.; Ismail, J. Synthesis of chitosan-stabilized platinum and palladium nanoparticles and their hydrogenation activity. J. Mol. Catal. A Chem. 2004, 212, 141–149. [Google Scholar] [CrossRef]
- Kumar, A.; Joshi, H.M.; Mandale, A.B.; Srivastava, R.; Adyanthaya, S.D.; Pasricha, R.; Sastry, M. Phase transfer of platinum nanoparticles from aqueous to organic solutions using fatty amine molecules. J. Chem. Sci. 2004, 116, 293–300. [Google Scholar] [CrossRef]
- Shukla, N.; Svedberg, E.B.; Ell, J. Surfactant isomerization and dehydrogenation of FePt nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 113–116. [Google Scholar] [CrossRef]
- Du, H.Y.; Wang, C.H.; Hsu, H.C.; Chang, S.T.; Chen, U.S.; Yen, S.C.; Chen, L.C.; Shih, H.C.; Chen, K.H. Controlled platinum nanoparticles uniformly dispersed on nitrogen-doped carbon nanotubes for methanol oxidation. Diam. Relat. Mater. 2008, 17, 535–541. [Google Scholar] [CrossRef]
- Bonnemann, H.; Richards, R.M. Nanoscopic metal particles—Synthetic methods and potential applications. Eur. J. Inorg. Chem. 2001, 2001, 2455–2480. [Google Scholar] [CrossRef]
- Liu, Z.L.; Lee, J.Y.; Han, M.; Chen, W.X.; Gan, L.M. Synthesis and characterization of PtRu/C catalysts from microemulsions and emulsions. J. Mater. Chem. 2002, 12, 2453–2458. [Google Scholar] [CrossRef]
- Kyung Tae, K.; Sung-Ho, J.; Seung-Cheol, C.; Deog-Su, P. Green Synthesis of Platinum Nanoparticles by Electroreduction of a K2PtCl6 Solid-State Precursor and Its Electrocatalytic Effects on H2O2 Reduction. Bull. Korean Chem. Soc. 2013, 34, 3835–3839. [Google Scholar]
- Zeng, J.; Yang, J.L.; Zhou, W. Activities of Pt/C catalysts prepared by low temperature chemical reduction methods. Appl. Catal. A 2006, 308, 99–104. [Google Scholar] [CrossRef]
- Meltzer, S.; Resch, R.; Koel, B.; Thompson, M.; Madhukar, A.; Requicha, A. Fabrication of nanostructures by hydroxylamine seeding of gold nanoparticle templates. Langmuir 2001, 17, 1713–1718. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Sadighikia, S.; Gursel, S.A. Scalable Synthesis of Sub-Nanosized Platinum-Reduced Graphene Oxide Composite by an Ultraprecise Photocatalytic Method. ACS Sustain. Chem. Eng. 2018, 6, 3773–3782. [Google Scholar] [CrossRef]
- Reetz, M.T.; Koch, M.G. Water-Soluble Colloidal Adams Catalyst: Preparation and Use in Catalysis. J. Am. Chem. Soc. 1999, 121, 7933–7934. [Google Scholar] [CrossRef]
- Du, Y.K.; Yang, P.; Mou, Z.G.; Hua, N.P.; Jiang, L. Thermal decomposition behaviors of PVP coated on platinum nanoparticles. J. Appl. Polym. Sci. 2006, 99, 23–26. [Google Scholar] [CrossRef]
- Okitsu, K.; Yue, A.; Tanabe, S.; Matsumoto, H. Sonochemical Preparation and Catalytic Behavior of Highly Dispersed palladium Nanoparticles on Alumina. Chem. Mater. 2000, 12, 3006–3011. [Google Scholar] [CrossRef]
- Fujimoto, T.; Terauchi, S.; Umehara, H.; Kojima, I.; Henderson, W. Sonochemical preparation of Single-Dispersion metal nanoparticles from metal salts. Chem. Mater. 2001, 13, 1057–1060. [Google Scholar] [CrossRef]
- Saminathan, K.; Kamavaram, V.; Veedu, V.; Kannan, A.M. Preparation and evaluation of electrodeposited platinum nanoparticles on in situ carbon nanotubes grown carbon paper for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 3838–3844. [Google Scholar] [CrossRef]
- Shafiei, M.; Riahi, A.R.; Sen, F.G.; Alpas, A.T. Improvement of platinum adhesion to carbon surfaces using PVD coatings. Surf. Coat. Technol. 2010, 205, 306–311. [Google Scholar] [CrossRef]
- Leong, G.J.; Schulze, M.C.; Strand, M.B.; Maloney, D.; Frisco, S.L.; Dinh, H.N.; Pivovar, B.; Richards, R.M. Shape-directed platinum nanoparticle synthesis: Nanoscale design of novel catalysts. Appl. Organomet. Chem. 2014, 28, 1–17. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Lim, S.I.; Ojea-Jime´nez, I.; Varon, M.; Casals, E.; Arbiol, J.; Puntes, V. Synthesis of platinum cubes, polypods, cuboctahedrons, and raspberries assisted by cobalt nanocrystals. Nano Lett. 2010, 10, 964–973. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Nakamura, S.; Miyake, M. Synthesis of small platinum cube with less than 3 nm by the control of growth kinetics. Cryst. Growth Des. 2011, 11, 4292–4295. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, C.M.; Solla-Gullon, J.; Vidal-Iglesias, F.J.; Aldaz, A.; Montiel, V.; Herrero, E. Imaging Structure Sensitive Catalysis on Different Shape-Controlled Platinum Nanoparticles. J. Am. Chem. Soc. 2010, 132, 5622–5624. [Google Scholar] [CrossRef] [PubMed]
- Formo, E.; Lee, E.; Campbell, D.; Xia, Y.N. Functionalization of Electrospun TiO2 Nanofibers with Pt Nanoparticles and Nanowires for Catalytic Applications. Nano Lett. 2008, 8, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.D.; Mandal, S.; Senapati, S.R.; Sainkar, M.; Khan, I. Bioreduction of AuCl4− Ions by the Fungus, Verticillium sp. and Surface Trapping of the Gold Nanoparticles Formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Nath, D.; Banerjee, P. Environ Green nanotechnology—A new hope for medical biology. Environ. Toxicol. Pharmacol. 2013, 36, 997–1014. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Riddin, T.; Gerickeb, M.; Whiteley, C.G. Biological synthesis of platinum nanoparticles: Effect of initial metal concentration. Enzyme Microb. Technol. 2010, 46, 501–505. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef]
- Gaidhani, S.V.; Yeshvekar, R.K.; Shedbalkar, U.U.; Bellare, J.H.; Chopade, B.A. Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process. Biochem. 2014, 49, 2313–2319. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Riddin, T.L.; Gericke, M.; Whiteley, C.G. Analysis of the inter-and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 2006, 17, 3482–3489. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M.; Whiteley, C.G. Bioreduction of platinum salts into nanoparticles: A mechanistic perspective. Biotechnol. Lett. 2009, 31, 95–100. [Google Scholar] [CrossRef]
- Syed, A.; Ahmad, A. Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concept and future applications. J. Nanopart. Res. 2007, 10, 507–517. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Moreno-Velasquez, S.D.; Vilchis-Nestor, A.R.; Berumen, E.A.; Borja, M.A. Production of Platinum Nanoparticles and Nanoaggregates Using Neurospora crassa. J. Microbiol. Biotechnol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and Growth of Au Nanoparticles inside live Alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess. Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R. Synthesis and structural characteristic of platinum nanoparticles using herbal bidens tripartitus extract. J. Inorg. Organomet. Polym. Mater. 2015, 26, 219–225. [Google Scholar] [CrossRef]
- Sheny, D.S.; Philip, D.; Mathew, J. Synthesis of platinum nanoparticles using dried Anacardium occidentale leaf and its catalytic and thermal applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Nancy, C.A. Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Rajasekharreddy, P.; Rani, P.U. Biosynthesis and characterization of Pd and Pt nanoparticles using Piper betle L. plant in a photoreduction method. J. Cluster Sci. 2014, 25, 1377–1388. [Google Scholar] [CrossRef]
- Soundarrajan, C.; Sankari, A.; Dhandapani, P.; Maruthamuthu, S.; Ravichandran, S.; Sozhan, G.; Palaniswamy, N. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess. Biosyst. Eng. 2012, 35, 827–833. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104–105114. [Google Scholar] [CrossRef]
- Gopi, K.J.R.; Panneerselvam, R. Quantification of colchicine in seed and tuber samples of Gloriosa superba by high performance liquid chromatography method. J. Appl. Pharm. Sci. 2011, 1, 116–119. [Google Scholar]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; Alessandro, N.D. One pot synthesis of lignin-stabilized platinum and palladium nanoparticles and their catalytic behaviours in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019, 12, 330–349. [Google Scholar] [CrossRef]
- Xiaobo, L.; Min, W.; Dayong, W.; Shigenori, K.; Takashi, E.; Yong, H. Platinum nanoparticles using wood nanomaterials: Green synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem. 2011, 13, 283. [Google Scholar]
- Musthafa, O.M.; Sudip, C.; Tasneem, A.; Shahid, A.A.A. Clean-Green Synthesis of Platinum Nanoparticles Utilizing a Pernicious Weed Lantana (Lantana camara). Am. J. Eng. Appl. Sci. 2016, 9, 84–90. [Google Scholar]
- Velmurugan, P.; Shim, J.; Kim, K.; Oh, B.T. Prunus × yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater. Lett. 2016, 174, 61–65. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Athinarayanan, J.; Subbarayan, P.V. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. Mater. Med. 2015, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, S.U.; Abbasi, T.; Abbasi, S.A. Biomimetic synthesis of platinum nanoparticles utilizing a terrestrial weed Antigonon leptopu. Part. Sci. Technol. 2017, 36, 681–688. [Google Scholar] [CrossRef]
- Rokade, S.; Joshi, K.A.; Mahajan, K.; Tomar, G.; Dubal, D.S.; Parihar, V.S.; Kitture, R.; Bellare, J.; Ghosh, S. Novel Anticancer Platinum and Palladium Nanoparticles from Barleria prionitis. Glob. J. Nanomed. 2017, 2, 555–600. [Google Scholar]
- Dauthal, P.; Mukhopadhyay, M. Biofabrication, characterization, and possible bio-reduction mechanism of platinum nanoparticles mediated by agro-industrial waste and their catalytic activity. J. Ind. Eng. Chem. 2015, 22, 185–191. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Sahin, B.; Aygün, A.; Gündüz, H.; Sahin, K.; Demir, E.; Akocak, S.; Sen, F. Cytotoxic Effects of Platinum Nanoparticles Obtained from Pomegranate Extract by the Green Synthesis Method on the MCF-7 Cell Line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. The Effects of Apigenin-Biosynthesized Ultra-Small Platinum Nanoparticles on the Human Monocytic THP-1 Cell Line. Cells 2019, 8, 444. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Differential immunomodulatory effect of graphene oxide and vanillin-functionalized graphene oxide nanoparticles in human acute monocytic leukemia cell line (THP-1). Int. J. Mol. Sci. 2019, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures and Nanomaterials. Synthesis, Properties, and Applications; Imperial College Press: London, UK, 2004. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale. Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin: Gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Perevedentseva, E.V.; Su, F.Y.; Su, T.H.; Lin, Y.C.; Cheng, C.L.; Karmenyan, A.V.; Priezzhev, A.V.; Lugovtsov, A.E. Laser-optical investigation of the effect of diamond nanoparticles on the structure and functional properties of proteins. Quantum Electron. 2010, 40, 1089–1093. [Google Scholar] [CrossRef]
- Charles, B.; Cher, M.T.; Jeng, C.K. FTIR spectroscopy as a tool for nano-material characterization. Inf. Biophys. Technol. 2010, 53, 434–438. [Google Scholar]
- Dzakpasu, R.; Axelrod, D. Dynamic light scattering microscopy. A novel optical technique to image submicroscopic motions. I: Theory. Biophys. J. 2004, 87, 1279–1287. [Google Scholar] [CrossRef]
- Digman, M.A.; Gratton, E. Lessons in fluctuation correlation spectroscopy. Annu. Rev. Phys. Chem. 2011, 62, 645–668. [Google Scholar] [CrossRef]
- Satoh, A.; Chantrell, R.W.; Brownian, G.N. Dynamics Simulations of Ferromagnetic Colloidal Dispersions in a Simple Shear Flow Coverdale. J. Colloid Interface Sci. 1999, 209, 44–59. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of nanoparticle by light scattering techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophy. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Cantor, C.R.; Schimmel, P.R.; Freeman Ed, W.H. Techniques for the Study of Biological Structure and Function; Standford Libraries, Standford Publishers: San Francisco, CA, USA, 1980. [Google Scholar]
- Waseda, Y.; Matsubara, E.; Shinoda, K.; Springer, V. X-Ray Diffraction Crystallography; Springer: Berlin, Germany, 2011. [Google Scholar]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, F.M.A.; Bazán-Díaz, L.; Mendoza-Cruz, R.; Gómez-Rodríguez, A.; Zorrilla-Cangas, C.; Herrera-Becerra, R. Nano Phase Characterization by Transmission Electron Microscopy: Experimental and Simulation. Mater. Sci. Appl. 2015, 6, 935. [Google Scholar] [CrossRef][Green Version]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Lyman, C.E.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron. Microscopy and X-Ray Microanalysis, 3rd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Bogner, A.; Jouneau, P.-H.; Thollet, G.; Basset, D.; Gauthier, C. A history of scanning electron microscopy developments: Towards “wet-STEM” imaging. Micron 2007, 38, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, P.W. The correction of electron lens aberrations. Ultramicroscopy 2015, 156, A1–A64. [Google Scholar] [CrossRef]
- Takenaka, S.; Karg, E.; Kreyling, W.G.; Lentner, B.; Möller, W.; BehnkeSemmler, M.; Jennen, L.; Walch, A.; Michalke, B.; Schramel, P.; et al. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal. Toxicol. 2006, 18, 733–740. [Google Scholar] [CrossRef]
- Beck-Speier, I.; Dayal, N.; Karg, E.; Maier, K.L.; Schumann, G.; Schulz, H.; Semmler, M.; Takenaka, S.; Stettmaier, K.; Bors, W.; et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free Radic. Biol, Med. 2005, 38, 1080–1092. [Google Scholar] [CrossRef]
- Stearns, R.C.; Paulauskis, J.D.; Godleski, J.J. Endocytosis of ultrafine particles by A549 cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 108–115. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabaté, S.; Krug, H. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Worcester, D.L.; Miller, R.G.; Bryant, P.J. Atomic force microscopy of purple membranes. J. Microsc. 1988, 152, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.; Vesenka, J.; Tang, C.L.; Rees, W.; Guthod, M.; Keller, R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry 1992, 31, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wiesendanger, R. Scanning Probe Microscopy and Spectroscopy; Cambridge University Press: Cambridge, UK, 1994; p. 659. ISBN 0521418100. [Google Scholar]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Valentine, J.S.; Gimzewski, J.K. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 2004, 305, 1147–1150. [Google Scholar] [CrossRef]
- Horber, J.K.; Miles, M.J. Scanning probe evolution in biology. Science 2003, 302, 1002–1005. [Google Scholar] [CrossRef]
- Charras, G.T.; Horton, M.A. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys. J. 2002, 82, 2970–2981. [Google Scholar] [CrossRef]
- Greenleaf, W.J.; Woodside, M.T.; Block, S.M. High-resolution, single-molecule measurements of biomolecular motion. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 171–190. [Google Scholar] [CrossRef]
- Dufrêne, Y.F. Using nanotechniques to explore microbial surfaces. Nat. Rev. Microbiol. 2004, 2, 2,451–460. [Google Scholar] [CrossRef]
- Rabinovich, Y.; Esayanur, M.; Daosukho, S.; Byer, K.; El-Shall, H.; Khan, S. Atomic force microscopy measurement of the elastic properties of the kidney epithelial cells. J. Colloid Interface Sci. 2005, 285, 125–135. [Google Scholar] [CrossRef]
- Osada, T.; Uehara, H.; Kim, H.; Ikai, A. Clinical laboratory implications of single living cell mRNA analysis. Adv. Clin. Chem. 2004, 38, 239–257. [Google Scholar]
- Uehara, H.; Osada, T.; Ikai, A. Quantitative measurement of mRNA at different loci within an individual living cell. Ultramicroscopy 2004, 100, 197–201. [Google Scholar] [CrossRef]
- Uehara, H.; Ikai, A.; Osada, T. Detection of mRNA in single living cells using AFM nanoprobes. Micro Nano Technol. Bioanal. 2009, 544, 599–608. [Google Scholar]
- Hu, M.; Wang, J.; Zhao, H.; Dong, S.; Cai, J. Nanostructure and nanomechanics analysis of lymphocyte using AFM: From resting, activated to apoptosis. J. Biomech. 2009, 42, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.; Scheuring, S.; Müller, S.A.; Engel, A.; Müller, D.J. Imaging and manipulation of biological structures with the AFM. Micron 2002, 33, 385–397. [Google Scholar] [CrossRef]
- Vlaic, G.; Olivi, L. EXAFS Spectroscopy: A Brief Introduction. Croat. Chem. Acta 2004, 77, 427–433. [Google Scholar]
- Kossel, W. Rontgenspektren. Z. Phys. 1920, 1, 119. [Google Scholar] [CrossRef]
- Kim, D.K.; Kan, D.; Veres, T.; Normadin, F.; Liao, J.K.; Kim, H.H.; Lee, S.-H.; Zahn, M.; Muhammed, M. Monodispersed Fe—Pt nanoparticles for biomedical applications. J. Appl. Phys. 2005, 97, 10–18. [Google Scholar] [CrossRef]
- Sun, S. Recent Advances in Chemical Synthesis, Self-Assembly, and Applications of FePt Nanoparticles. Adv. Mater. 2006, 18, 393–403. [Google Scholar] [CrossRef]
- Chiang, P.C.; Hung, D.S.; Wang, J.W.; Ho, C.S.; Yao, Y.D. Engineering Water-Dispersible FePt Nanoparticles for Biomedical Applications. IEEE Trans. Magn. 2007, 43, 2445. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, M.; Jiang, X.; Liang, S. Recent Advances in FePt Nanoparticles for Biomedicine. J. Nanomater. 2015, 2015, 13. [Google Scholar] [CrossRef]
- Maenosono, S.; Saita, S. Theoretical Assessment of FePt Nanoparticles as Heating Elements for Magnetic Hyperthermia. IEEE Trans. Magn. 2006, 42, 1638. [Google Scholar] [CrossRef]
- Seehra, M.S.; Singh, V.; Dutta, P.; Neeleshwar, S.; Chen, Y.Y.; Chen, C.L.; Chou, S.W.; Chen, C.C. Size-dependent magnetic parameters of fcc FePt nanoparticles: Applications to magnetic hyperthermia. J. Phys. D Appl. Phys. 2010, 43, 145002. [Google Scholar] [CrossRef]
- Maenosono, S.; Suzuki, T.; Saita, S. Superparamagnetic FePt nanoparticles as excellent MRI contrast agents. J. Magn. Magn. Mater. 2008, 320, L79–L83. [Google Scholar] [CrossRef]
- Taylor, R.M.; Sillerud, L.O. Paclitaxel-loaded iron platinum stealth immunomicelles are potent MRI imaging agents that prevent prostate cancer growth in a PSMA-dependent manner. Int. J. Nanomed. 2012, 7, 4341–4352. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Tian, Q. One-pot synthesis of amphiphilic superparamagnetic FePt nanoparticles and magnetic resonance imaging in vitro. J. Magn. Magn. Mater. 2010, 322, 973–977. [Google Scholar] [CrossRef]
- Chou, S.W.; Shau, Y.H.; Wu, P.C.; Yang, Y.S.; Shieh, D.B.; Chen, C.C. In Vitro and in Vivo Studies of FePt Nanoparticles for Dual Modal CT/MRI Molecular Imaging. J. Am. Chem. Soc. 2010, 13238, 13270–13278. [Google Scholar] [CrossRef]
- Lai, S.M.; Tsai, T.Y.; Hsu, C.Y.; Tsai, J.L.; Liao, M.Y.; Lai, P.S. Bifunctional silica-coated superparamagnetic FePt nanoparticles for fluorescence/MR dual imaging. J. Nanomater. 2012, 2012, 631584. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Q.; Wang, M.; Zhu, Y.; Wu, Q.; Yang, X. Water-soluble l-cysteine-coated FePt nanoparticles as dual MRI/CT imaging contrast agent for glioma. Int. J. Nanomed. 2015, 10, 2325–2333. [Google Scholar]
- Fuchigami, T.; Kawamura, R.; Kitamoto, Y.; Nakagawa, M.; Namiki, Y. A magnetically guided anti-cancer drug delivery system using porous FePt capsules. Biomaterials 2012, 33, 1682–1687. [Google Scholar] [CrossRef]
- Chen, C.-L.; Kuo, L.-R.; Lee, S.-Y. Photothermal cancer therapy via femtosecond-laser-excited FePt nanoparticles. Biomaterials 2013, 34, 1128–1134. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Fan, T.; Zhan, H.; Wang, X.; Zhou, Y.; Yang, R. Targeted hyperthermia after selective embolization with ferromagnetic nanoparticles in a VX2 rabbit liver tumor model. Int. J..Nanomed. 2013, 8, 3795–3804. [Google Scholar] [CrossRef][Green Version]
- Seemann, K.M.; Luysberg, M.; Révay, Z. Magnetic heating properties and neutron activation of tungsten-oxide coated biocompatible FePt core-shell nanoparticles. J. Control. Release 2015, 197, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tang, Y.; Bao, Z.; Wang, H.; Ren, F.; Guo, M.; Quan, H.; Jiang, C. FePt nanoparticles as a potential X-ray activated chemotherapy agent for HeLa cells. Int. J. Nanomed. 2015, 10, 64356–66444. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, N.; Leung, K.T. FePt alloy nanoparticles for biosensing: Enhancement of vitamin C sensor performance and selectivity by nanoalloying. Anal. Chem. 2013, 85, 5974–5980. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Mitura, S.; Mitura, K.; Grodzik, M.; Orlowski, P.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomed. 2010, 5, 1085–1094. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Ma, S.; Izutani, N.; Imazato, S.; Chen, J.; Kiba, W.; Yoshikawa, R.; Takeda, K.; Kitagawa, H.; Ebisu, S. Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent. Mater. J. 2012, 31, 150–156. [Google Scholar] [CrossRef]
- Boomi, P.; Prabu, H.G.; Mathiyarasu, J. Synthesis and characterization of polyaniline/Ag-Pt nanocomposite for improved antibacterial activity. Colloids Surf. B 2013, 103, 9–14. [Google Scholar] [CrossRef]
- Elhusseiny, A.F.; Hassan, H.H. Antimicrobial and antitumor activity of platinum and palladium complexes of novel spherical aramides nanoparticles containing flexibilizing linkages: Structure–property relationship. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2013, 103, 232–245. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the Composition of AuPt Bimetallic Nanoparticles for Antibacterial Application. Angew. Chem. 2014, 126, 8265–8269. [Google Scholar] [CrossRef]
- Judy, G.; Nazim, H.; Manikandan, M.; Hui-Fen, W. Bacterial toxicity/compatibility of platinum nanospheres, nanocuboids and nanoflowers. Sci. Rep. 2013, 3, 1260. [Google Scholar]
- Taglietti, A.; Diaz Fernandez, Y.A.; Amato, E.; Cucca, L.; Dacarro, G.; Grisoli, P.; Necchi, V.; Pallavicini, P.; Pasotti, L.; Patrini, M. Antibacterial activity of glutathione-coated silver nanoparticles against Gram positive and Gram negative bacteria. Langmuir 2012, 28, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Raman, T.; Anbazhagan, V. Platinum nanoparticles inhibit bacteria proliferation and rescue zebrafish from bacterial infection. RSC Adv. 2016, 6, 44415. [Google Scholar] [CrossRef]
- Boomi, P.; Gurumallesh Prabu, H.; Mathiyarasu, J. Synthesis, characterization and antibacterial activity of polyaniline/Pt-Pd nanocomposite. Eur. J. Med. Chem. 2014, 72, 18–25. [Google Scholar] [CrossRef]
- Nam, K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J. Adv. Prosthodont. 2014, 3, 207–214. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Prakash, S.; Ahila, N.K.; Vinoj, G.; Selvam, S.; Kumar, G.; Kannapiran, E.; Babu Rajendran, R. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed. Pharmacother. 2017, 92, 479–490. [Google Scholar] [CrossRef]
- Deepika, G.; Sashidhar, R.B. Biopolymer-mediated synthesis and characterization of platinum nanocomposite and its anti-fungal activity against A. parasiticus and A. flavus. Micro Nano Lett. 2018, 13, 1491–1496. [Google Scholar]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef]
- Kelloff, G.J. Perspectives on cancer chemoprevention research and drug development. Adv. Cancer Res. 2000, 78, 199–334. [Google Scholar] [PubMed]
- Goodsell, D.S. The molecular perspective: Cisplatin. Stem Cells 2006, 24, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N. SEER Cancer Statistics Review, 1975–2009; National Cancer Institute: Bethesda, MD, USA, 2012.
- Pelka, J.; Gehrke, H.; Esselen, M.; Turk, M.; Crone, M.; Brase, S.; Muller, T.; Blank, H.; Send, W.; Zibat, V.; et al. Cellular uptake of platinum nanoparticles in human colon carcinoma cells and their impact on cellular redox systems and DNA integrity. Chem. Res. Toxicol. 2009, 22, 649–659. [Google Scholar] [CrossRef]
- Cemazar, M.; Milacic, R.; Miklavcic, D.; Dolzan, V.; Sersa, G. Intratumoral cisplatin administration in electro chemotherapy: Antitumor effectiveness, sequence dependence and platinum content. Anticancer Drugs 1998, 9, 525–530. [Google Scholar] [CrossRef][Green Version]
- Getaz, E.P.; Beckley, S.; Fitzpatrick, J.; Dozier, A. Cisplatin Induced Hemolysis. N. Engl. J. Med. 1980, 302, 334–335. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. J. Inhal. Toxicol. 2008, 9, 575–583. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Comenge, J.; Sotelo, C.; Romero, F.; Gallego, O.; Barnadas, A.; Parada, T.G.C.; Domínguez, F.; Puntes, V.F. Detoxifying Antitumoral Drugs via Nanoconjugation: The Case of Gold Nanoparticles and Cisplatin. PLoS ONE 2012, 7, e47562. [Google Scholar] [CrossRef]
- Rousseau, J.; Barth, R.F.; Fernandez, M.; Adam, J.F.; Balosso, J.; Estève, F.; Elleaume, H. Efficacy of intracerebral delivery of cisplatin in combination with photon irradiation for treatment of brain tumors. J. Neuro Oncol. 2010, 8, 287–295. [Google Scholar] [CrossRef][Green Version]
- Silici, S.; Ekmekcioglu, O.; Kanbur, M.; Deniz, K. The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J. Urol. 2011, 8, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wakai, S.; Hirokawa, N. Development of the blood-brain barrier to horseradish peroxidase in the chick embryo. Cell Tissue Res. 1978, 8, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 8, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Prasek, M.; Sawosz, E.; Jaworski, S.; Grodzik, M.; Ostaszewska, T.; Kamaszewski, M.; Wierzbicki, M.; Chwalibog, A. Influence of nanoparticles of platinum on chicken embryo development and brain morphology. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. Nanotechnology 2014. [Google Scholar] [CrossRef]
- Borse, V.; Kaler, A.; Banerjee, U.C. Microbial synthesis of platinum nanoparticles and evaluation of their anticancer activity. Int. J. Emerg. Trends Electr. Electron. 2015, 11, 26–31. [Google Scholar]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopad, B.A. Novel platinum-palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7490. [Google Scholar]
- Bendale, Y.; Bendale, V.; Natu, R.; Paul, S. Biosynthesized Platinum Nanoparticles Inhibit the Proliferation of Human Lung-Cancer Cells in vitro and Delay the Growth of a Human Lung-Tumor Xenograft in vivo—In vitro and in vivo Anticancer Activity of bio-Pt NPs. J. Pharmacopunct. 2016, 19, 114–121. [Google Scholar]
- Medhat, A.; Mansour, S.; El-sonbaty, S.; Kandil, E.; Mahmoud, M. Evaluation of the antitumor activity of platinum nanoparticles in the treatment of hepatocellular carcinoma induced in rats. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Hinzmann, M.; Wierzbicki, M.; Hotowy, A.; Grodzik, M.; Winnicka, A.; Chwalibog, A. Investigation of platinum nanoparticle properties against U87 glioblastoma multiforme. Arch. Med. Sci. 2017, 6, 1322–1334. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Graphene Oxide—Platinum Nanoparticle Nanocomposites: A Suitable Biocompatible Therapeutic Agent for Prostate Cancer. Polymers 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Jokerst, J.; Zavaleta, C.; Massoud, T.F.; Gambhir, S.S. Gold nanoparticles:A Revival in precious Metal Administration to Patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A. Platinum in silicone breast implants. Biomaterials 2006, 27, 3274–3286. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Manikandan, M.; Hasan, N.; Wu, H.F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013, 34, 5833–5842. [Google Scholar] [CrossRef]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Koayashi, K.; Furusawa, Y.; Lesech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 085103. [Google Scholar] [CrossRef]
- Shvedova, A.; Pietroiusti, A.; Kagan, V. Nanotoxicology ten years later: Lights and shadows. Toxicol. Appl. Pharmacol. 2016, 299, 1–2. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Sukhanova, I.N. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Elder, A.; Yang, H.; Gwiazda, R.; Teng, X.; Thurston, S.; He, H.; Oberdorster, G. Testing Nanomaterials of Unknown Toxicity: An Example Based on Platinum Nanoparticles of Different Shapes. Adv. Mater. 2007, 19, 3124–3129. [Google Scholar] [CrossRef]
- Kostova, I. Platinum complexes as anticancer agents. Recent Pat. Anticancer Drug Discov. 2006, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, S.; Aoshiba, K.; Kajita, M.; Miyamoto, Y.; Nagai, A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. 2009, 22, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liang, G.; Zhang, B.; Kuang, Y.; Zhang, X.; Xu, B. FePt@CoS(2) yolk-shell nanocrystals as a potent agent to kill HeLa cells. J. Am. Chem. Soc. 2007, 129, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Teow, Y.; Valiyaveettil, S. Active targeting of cancer cells using folic acid-conjugated platinum nanoparticles. Nanoscale 2010, 2, 2607–2613. [Google Scholar] [CrossRef]
- Asharani, P.V.; Xinyi, N.; Hande, M.P.; Valiyaveettil, S. DNA damage and p53-mediated growth arrest in human cells treated with PtNPs. Nanomedicine 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Konieczny, P.; Anna, G.G.; Skalniak, L.; Koziel, J.; Francesca, L.F.; Crosera, M.; Cierniak, A.; Zuba, S.; Borowczyk, J.; Laczna, E.; et al. Effects triggered by platinum nanoparticles on primary keratinocytes. Int. J. Nanomed. 2013, 8, 3963–3975. [Google Scholar]
- Hashimoto, M.; Kawai, K.; Kawakami, H.; Imazato, S. Matrix metalloproteases inhibition and biocompatibility of gold and platinum nanoparticles. J. Biomed. Mater. Res. Part. A 2016, 104, 209–217. [Google Scholar] [CrossRef]
- Subramaniyan, S.B.; Ramani, A.; Ganapathi, V.; Anbazhan, V. Preparation of self-assembled platinum nanoclusters to combat Salmonella typhi infection and inhibit biofilm formation. Colloids Surf. B Biointerfaces 2018, 171, 75–84. [Google Scholar] [CrossRef]
- Loan, T.T.; Do, L.T.; Yoo, H. Platinum Nanoparticles Induce Apoptosis on Raw 264.7 Macrophage Cells. J. Nanosci. Nanotechnol. 2018, 18, 861–864. [Google Scholar] [CrossRef]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Wei, H. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green Platinum Nanoparticles Interaction With HEK293 Cells: Cellular Toxicity, Apoptosis, and Genetic Damage. Dose Response 2018, 16, 1559325818807382. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, M.S.; Vonderach, T.; Hattendorf, B.; Wennemers, H. Peptide-Coated Platinum Nanoparticles with Selective Toxicity against Liver Cancer Cells. Angew. Chem. 2019, 58, 4901–4905. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okinaga, T.; Iwanaga, K.; Matsuo, K.; Toyono, T.; Sasaguri, M.; Ariyoshi, W.; Tominaga, K.; Enomoto, Y.; Matsumura, Y.; et al. Anticancer effect of novel platinum nanocomposite beads on oral squamous cell carcinoma cells. J. Biomed. Mater. Res. Part. B 2019, 107, 2281–2287. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Lianwu, Y.; Gong, Z.; Valiyaveettil, S. Comparison of the toxicity of silver, gold and PtNPs in developing zebrafish embryos. Nanotoxicology 2011, 5, 43–54. [Google Scholar] [CrossRef]
- Labrador-Rached, C.J.; Browning, R.T.; Braydich-Stolle, L.K.; Comfort, K.K. Toxicological Implications of Platinum Nanoparticle Exposure: Stimulation of Intracellular Stress, Inflammatory Response, and Akt Signaling In Vitro. J. Toxicol. 2018, 136, 7801. [Google Scholar] [CrossRef]
- Gehrke, H.; Pelka, J.; Hartinger, C.G.; Blank, H.; Bleimund, F.; Schneider, R.; Gerthsen, D.; Brase, S.; Crone, M.; Turk, M.; et al. Platinum nanoparticles and their cellular uptake and DNA platination at non-cytotoxic concentrations. Arch. Toxicol. 2011, 85, 799–812. [Google Scholar] [CrossRef]
- Mironava, T.; Simon, M.; Rafailovich, M.H.; Rigas, B. Platinum folate nanoparticles toxicity: Cancer vs. normal cells. Toxicol. Vitr. 2013, 27, 882–889. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Watari, A.; Hayata, Y.; Li, X.; Kondoh, M.; Tsutsumi, Y.; Yagi, K. Hepatotoxicity of sub-nanosized platinum particles in mice. Pharmazie 2013, 68, 178–182. [Google Scholar]
- Ho, Y.P.; Au-Yeung, S.C.; To, K.K. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med. Res. Rev. 2003, 23, 633–655. [Google Scholar] [CrossRef]
- Hamelers, I.H.; Staffhorst, R.W.; Voortman, J.; de Kruijff, B.; Reedijk, J.; en Henegouwen, P.M.V.B.; de Kroon, A.I. High Cytotoxicity of Cisplatin Nanocapsules in Ovarian Carcinoma Cells Depends on Uptake by Caveolae-Mediated Endocytosis. Clin. Cancer Res. 2009, 15, 2009. [Google Scholar] [CrossRef]
- Liu, L.; Miao, P.; Xu, Y.; Tian, Z.; Zou, Z.; Li, G. Study of Pt/TiO2 nanocomposite for cancer-cell treatment. J. Photochem. Photobiol. B Biol. 2010, 98, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, M.; Wang, J.; Tan, F.; Li, N. The mitochondria-targeted and IR780-regulated theranosomes for imaging and enhanced photodynamic/photothermal therapy. RSC Adv. 2016, 6, 11070–11076. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Bui, N.Q.; Moorthy, M.S.; Lee, K.D.; Oh, J. Synthesis and In Vitro Performance of Polypyrrole-Coated Iron–Platinum Nanoparticles for Photothermal Therapy and Photoacoustic Imaging. Nano. Res. Lett. 2017, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, H.; Ma, S.; Zhang, L.; You, C.; Tang, F.; Yang, C.; Tian, X.; Wang, F.; Luo, Y.; et al. FePt-Cys nanoparticles induce ROS-dependent cell toxicity, and enhance chemo-radiation sensitivity of NSCLC cells in vivo and in vitro. Cancer Lett. 2018, 418, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ankkeev, V.; Maohong, F.I. Supercritical Fluid Technology for Energy and Environmental Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Wang, X.; Guo, Z. Targeting and delivery of platinum based anticancer drugs. Chem. Soc. Rev. 2013, 42, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Matsumura, Y.; Maeda, H.A. New concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; di Tomaso, E.; Jain, R.K. Pathology: Cancer cells compress intratumour vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Nishiyama, N.; Kataoka, K. Optimization of (1,2-diamino-cyclohexane) platinum (II)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity. J. Control. Release 2007, 121, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Boulikas, T.; Stathopoulos, G.P.; Volakakis, N.; Vougiouka, M. Systemic Lipoplatin infusion results in preferential tumor uptake in human studies. Anticancer Res. 2005, 25, 3031–3039. [Google Scholar]
- Steinhauser, I.; Spankuch, B.; Strebhardt, K.; Langer, K. Trastuzumab-modified nanoparticles: Optimisation of preparation and uptake in cancer cells. Biomaterials 2006, 27, 4975–4983. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Matherly, L.H.; Goldman, D.I. Membrane transport of folates. Vitam. Horm. 2003, 66, 403–456. [Google Scholar]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nano-carriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298. [Google Scholar] [CrossRef]
- Sudimack, J.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2000, 41, 147–162. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt (IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hong, H.; Cai, W. Tumor-targeted drug delivery with aptamers. Curr. Med. Chem. 2011, 18, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Dhar, S.; Kolishetti, N.; Lippard, S.J.; Farokhzad, O.C. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 1850–1855. [Google Scholar] [CrossRef]
- Leo, A.J.; Oluwafemi, O.S. Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source—An eco-friendly development. Mater. Lett. 2017, 196, 141–144. [Google Scholar]
- Karthik, R.; Sasikumar, R.; Chen, S.M.; Govindasamy, M.; Kumar, J.V.; Muthuraj, V. Green synthesis of Platinum Nanoparticles Using Quercus Glauca Extract and its electrochemical oxidation of Hydrazine in water samples. Int. J. Electrochem. Sci. 2016, 11, 8245–8255. [Google Scholar] [CrossRef]
- Nellore, J.; Pauline, C.; Amarnath, K. Bacopa monnieri Phytochemicals Mediated Synthesis of Platinum Nanoparticles and Its Neurorescue Effect on 1-Methyl 4-Phenyl 1,2,3,6 Tetrahydropyridine-Induced Experimental Parkinsonism in Zebrafish. J. Neurodegener. Dis. 2013. [Google Scholar] [CrossRef]
- Vinod, V.T.P.; Saravanan, P.; Sreedhar, B.; Devi, D.K.; Sashidhar, R. A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf. B Biointerfaces 2011, 83, 291–298. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum (IV) chloride complex. Langmuir 2006, 22, 7318–7323. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djedjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Velmurugan, P.; Park, J.H.; Lovanh, N.; Seo, S.K.; Jayanthi, P.; Park, Y.J.; Cho, M.; Oh, B.T. Synthesis and antimicrobial activity of palladium nanoparticles from Prunus x yedoensis leaf extract. Mater. Lett. 2016, 185, 335–338. [Google Scholar] [CrossRef]
- Rokade, S.S.; Komal, A.J.; Mahajan, K.; Patil, S.; Tomar, G.; Dubal, D.S.; V Parihar, V.S.; Kitture, R.; Bellare, J.R.; Ghosh, S. Gloriosa superba Mediated Synthesis of Platinum and Palladium Nanoparticles for Induction of Apoptosis in Breast Cancer. Bioinorg. Chem. Appl. 2018, 2018. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Tammina, S.K. Green synthesis of nano platinum using naturally occurring polyphenols. RSC Adv. 2013, 3, 4033–4039. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. https://doi.org/10.3390/nano9121719
Jeyaraj M, Gurunathan S, Qasim M, Kang M-H, Kim J-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials. 2019; 9(12):1719. https://doi.org/10.3390/nano9121719
Chicago/Turabian StyleJeyaraj, Muniyandi, Sangiliyandi Gurunathan, Muhammad Qasim, Min-Hee Kang, and Jin-Hoi Kim. 2019. "A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles" Nanomaterials 9, no. 12: 1719. https://doi.org/10.3390/nano9121719
APA StyleJeyaraj, M., Gurunathan, S., Qasim, M., Kang, M.-H., & Kim, J.-H. (2019). A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials, 9(12), 1719. https://doi.org/10.3390/nano9121719





