Preparation of a Pd/Al2O3 Catalyst with Microwave-Induced Plasma Jet Irradiation under Atmospheric Pressure
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Setup
2.2. Sample Preparation
- <Step 1> Al(OH)3 powder (gibbsite) was added into 5 wt% of Pd(NO3)2 solution.
- <Step 2> The solution was dried at 80 °C until it became slurry-like.
- <Step 3> The slurry was dried at 110 °C for 12 h and crushed.
- <Step 4 (PF)> The crushed particles were treated in the plasma using the fixed bed under the following plasma conditions: power = 270 W, Ar flow rate = 2.5–4 L/min, H2 flow rate = 60 mL/min, and treatment time = 15 min.
- <Step 4 (PS)> The crushed particles were treated using the plasma spouted bed reactor with the same plasma conditions as those used in PF.
- <Step 4 (CM)> In the same way as in PF, the crushed particles were heated to 500 °C or 900 °C using the electric furnace with a 16.7% H2/Ar mixture for 2 h.
2.3. Evaluation of the Pd/Al2O3 Catalyst: Selective Hydrogenation of Acetylene to Ethylene or Ethane
2.4. Catalyst Characterization
3. Results and Discussion
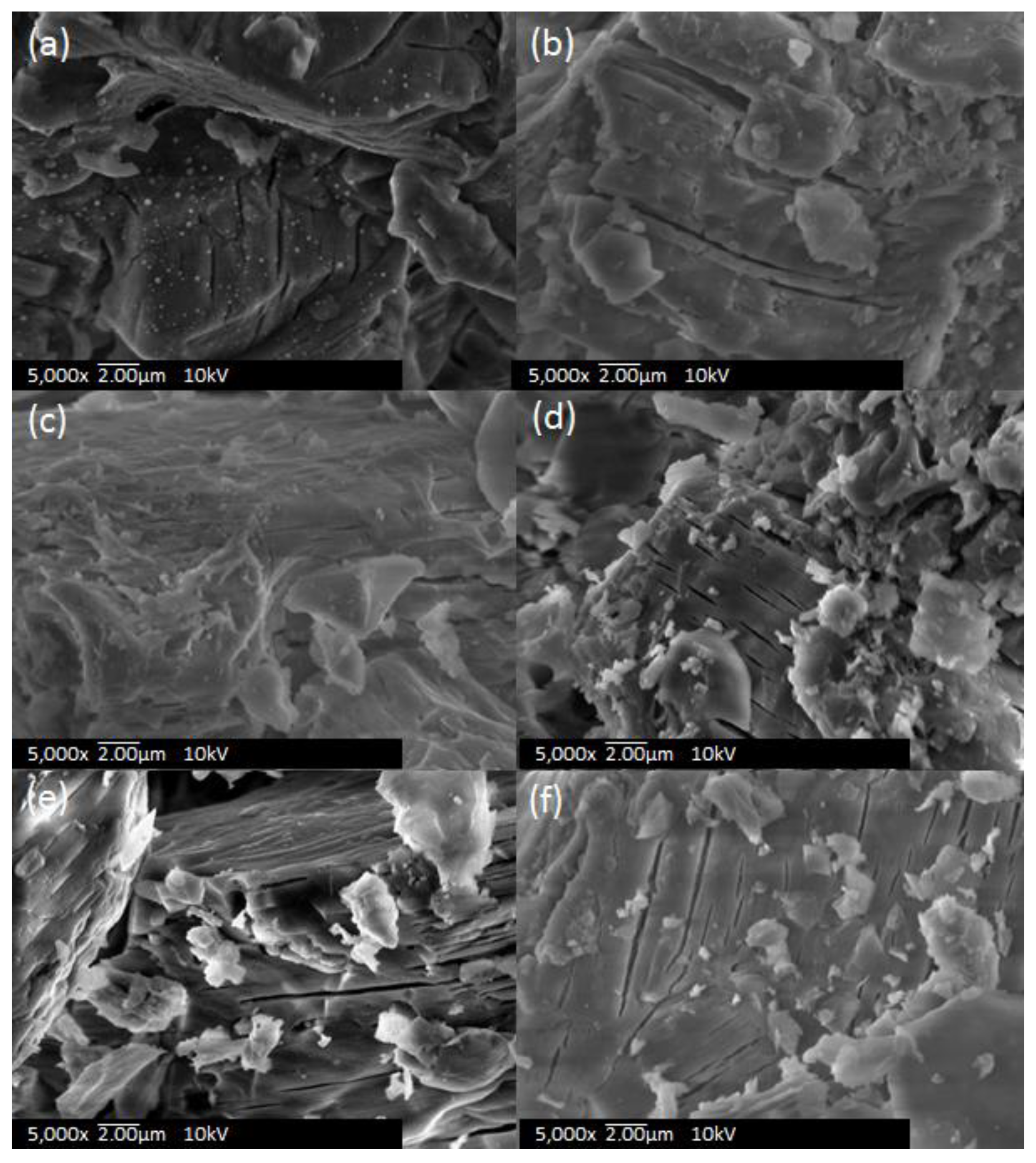
3.1. SEM
3.2. XRD
3.3. TEM
3.4. Acetylene Conversion
3.5. Ethylene and Ethane Selectivity
3.6. Comparison of the Fixed Bed with Plasma Irradiation and the Plasma Spouted Bed
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.J.; Vissokov, G.P.; Jang, B.W.L. Catalyst preparation using plasma technologies. Catal. Today 2002, 72, 173–184. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.L.; Liu, C.J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
- Fu, T.; Huang, C.; Lv, J.; Li, Z. Fuel production through Fischer–Tropsch synthesis on carbon nanotubes supported Co catalyst prepared by plasma. Fuel 2014, 121, 225–231. [Google Scholar]
- Jin, L.; Li, Y.; Lin, P.; Hu, H. CO2 reforming of methane on Ni/γ-Al2O3 catalyst prepared by dielectric barrier discharge hydrogen plasma. Int. J. Hydrog. Energy 2014, 39, 5756–5763. [Google Scholar] [CrossRef]
- Foix, M.; Guyon, C.; Tatoulian, M.; Da Costa, P. Microwave plasma treatment for catalyst preparation: Application to alumina supported silver catalysts for SCR NOx by ethanol. Mod. Res. Catal. 2013, 2, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zhao, B.; Liu, Y.; Li, Y. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective. Catal. Today 2015, 256, 29–40. [Google Scholar] [CrossRef]
- Hinokuma, S.; Fujii, H.; Katsuhara, Y.; Ikeue, K.; Machida, M. Effect of thermal ageing on the structure and catalytic activity of Pd/CeO2 prepared using arc-plasma process. Catal. Sci. Technol. 2014, 4, 2990–2996. [Google Scholar] [CrossRef]
- Kang, J.H.; Shin, E.W.; Kim, W.J.; Park, J.D.; Moon, S.H. Selective hydrogenation of acetylene on TiO2-added Pd catalysts. J. Catal. 2002, 208, 310–320. [Google Scholar]
- Sárkány, A.; Weiss, A.H.; Guczi, L. Structure sensitivity of acetylene-ethylene hydrogenation over Pd catalysts. J. Catal. 1986, 98, 550–553. [Google Scholar] [CrossRef]
- Doyle, A.M.; Shaikhutdinov, S.K.; Freund, H.J. Surface-Bonded Precursor Determines Particle Size Effects for Alkene Hydrogenation on Palladium. Angew. Chem. Int. Ed. 2005, 44, 629–631. [Google Scholar]
- Jin, M.; Zhang, H.; Xie, Z.; Xia, Y. Palladium nanocrystals enclosed by {100} and {111} facets in controlled proportions and their catalytic activities for formic acid oxidation. Energy Environ. Sci. 2012, 5, 6352–6357. [Google Scholar] [CrossRef] [Green Version]
- Shao, M.; Yu, T.; Odell, J.H.; Jin, M.; Xia, Y. Structural dependence of oxygen reduction reaction on palladium nanocrystals. Chem. Commun. 2011, 47, 6566–6568. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Kodama, S.; Sekiguchi, H. Preparation of catalyst with microwave induced plasma jet combined with spouted bed. J. Nanosci. Nanotechnol. 2019, 19, 6849–6855. [Google Scholar] [CrossRef] [PubMed]
- Flamant, G. Hydrodynamics and heat transfer in a plasma spouted bed reactor. Plasma Chem. Plasma Process. 1990, 10, 71–85. [Google Scholar] [CrossRef]
- Uglov, A.A.; Gnedovets, A.G. Effect of particle charging on momentum and heat transfer from rarefied plasma flow. Plasma Chem. Plasma Process. 1991, 11, 251–267. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, H.; Li, H.; Xu, X.; Liu, F.; Guo, S.; Xu, P. The effect of plasma-gas swirl flow on a highly constricted plasma cutting arc. J. Phys. D Appl. Phys. 2009, 42, 095208. [Google Scholar] [CrossRef]
- Puskas, I.; Cerefice, S.A. Process for Preparing Palladium on Carbon Catalysts for Purification of Crude Terephthalic Acid. U.S. Patent No. 4,476,242, 9 October 1984. [Google Scholar]
- Didillon, B.; Cameron, C.; Gautreau, C. Selective Hydrogenation Catalysts Containing Palladium, also Tin and/or Lead, and the Preparation and Use Thereof. U.S. Patent No. 5,955,397, 21 September 1999. [Google Scholar]
- Cider, L.; Schöön, N.H. Competition between ethyne, ethene and carbon monoxide for the active sites during hydrogenation at transient conditions over supported metal catalysts. Appl. Catal. 1991, 68, 191–205. [Google Scholar] [CrossRef]
- Asplund, S. Coke formation and its effect on internal mass transfer and selectivity in Pd-catalysed acetylene hydrogenation. J. Catal. 1996, 158, 267–278. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, C.; Yu, Q. Low-temperature direct current glow discharges at atmospheric pressure. IEEE Trans. Plasma Sci. 2005, 33, 328–329. [Google Scholar] [CrossRef]
- Ingram-Jones, V.J.; Slade, R.C.; Davies, T.W.; Southern, J.C.; Salvador, S. Dehydroxylation sequences of gibbsite and boehmite: Study of differences between soak and flash calcination and of particle-size effects. J. Mater. Chem. 1996, 6, 73–79. [Google Scholar] [CrossRef]
- Whittington, B.; Ilievski, D. Determination of the gibbsite dehydration reaction pathway at conditions relevant to Bayer refineries. Chem. Eng. J. 2004, 98, 89–97. [Google Scholar] [CrossRef]
- Perander, L. Evolution of Nano-and Microstructure During the Calcination of Bayer Gibbsite to Produce Alumina. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 2010. [Google Scholar]
- Chauruka, S.R.; Hassanpour, A.; Brydson, R.; Roberts, K.J.; Ghadiri, M.; Stitt, H. Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem. Eng. Sci. 2015, 134, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Kostić, E.; Kiss, S.J.; Zec, S.; Bošković, S. Transition of γ-Al2O3 into α-Al2O3 during vibro milling. Powder Technol. 2000, 107, 48–53. [Google Scholar] [CrossRef]
- ZielińAski, P.A.; Schulz, R.; Kaliaguine, S.; Van Neste, A. Structural transformations of alumina by high energy ball milling. J. Mater. Res. 1993, 8, 2985–2992. [Google Scholar] [CrossRef]
- Šepelák, V.; Indris, S.; Heitjans, P.; Becker, K.D. Direct determination of the cation disorder in nanoscale spinels by NMR, XPS, and Mössbauer spectroscopy. J. Alloy Compd. 2007, 434, 776–778. [Google Scholar] [CrossRef]
- Xiong, Y.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Akita, T.; Lu, P.; Ichikawa, S.; Tanaka, K.; Haruta, M. Analytical TEM study on the dispersion of Au nanoparticles in Au/TiO2 catalyst prepared under various temperatures. Surf. Interface Anal. 2001, 31, 73–78. [Google Scholar] [CrossRef]
- Liu, C.J.; Yu, K.; Zhang, Y.P.; Zhu, X.; He, F.; Eliasson, B. Characterization of plasma treated Pd/HZSM-5 catalyst for methane combustion. Appl. Catal. B: Environ. 2004, 47, 95–100. [Google Scholar] [CrossRef]
- Rahmani, F.; Haghighi, M.; Estifaee, P. Synthesis and characterization of Pt/Al2O3–CeO2 nanocatalyst used for toluene abatement from waste gas streams at low temperature: Conventional vs. plasma–ultrasound hybrid synthesis methods. Microporous Mesoporous Mater. 2014, 185, 213–223. [Google Scholar] [CrossRef]
- Zhu, X.; Huo, P.; Zhang, Y.P.; Cheng, D.G.; Liu, C.J. Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl. Catal. B: Environ. 2008, 81, 132–140. [Google Scholar] [CrossRef]
- Ge, C.; Fang, G.; Shen, X.; Chong, Y.; Wamer, W.G.; Gao, X.; Yin, J.J. Facet energy versus enzyme-like activities: The unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano 2016, 10, 10436–10445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jang, B.W.L. Selective hydrogenation of acetylene over Pd/Al2O3 catalysts: Effect of non-thermal RF plasma preparation methodologies. Top. Catal. 2017, 60, 997–1008. [Google Scholar] [CrossRef]





| Catalyst | Power (W) | Distance 1 (cm) | Ar Flow Rate (L/min) | H2 Flow Rate (mL/min) | Treatment Time (min) | Mass (g) |
|---|---|---|---|---|---|---|
| PF-1 | 270 | 3.0 | 2.5 | 60 | 7 | 1.0 |
| PF-2 | 270 | 5.0 | 2.5 | 60 | 15 | 1.0 |
| PF-3 | 270 | 7.0 | 2.5 | 60 | 15 | 1.0 |
| PS | 270 | 1.0 | 2.5 | 60 | 15 | 1.0 |
| Catalyst | Avg. Crystallite Size of Pd (Å) | Metal Surface Area (m²/g Sample) | Dispersion 1 (%) |
|---|---|---|---|
| PF-1 | 14.8 | 1.13 | 25.3 |
| PF-2 | 16.2 | 1.02 | 22.9 |
| PF-3 | 25.5 | 0.65 | 14.8 |
| PS | 16.2 | 1.03 | 23.2 |
| CM-500 | 19.2 | 0.86 | 19.6 |
| CM-900 | 22.1 | 0.75 | 17.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.Y.; Kodama, S.; Sekiguchi, H. Preparation of a Pd/Al2O3 Catalyst with Microwave-Induced Plasma Jet Irradiation under Atmospheric Pressure. Nanomaterials 2019, 9, 1734. https://doi.org/10.3390/nano9121734
Chung JY, Kodama S, Sekiguchi H. Preparation of a Pd/Al2O3 Catalyst with Microwave-Induced Plasma Jet Irradiation under Atmospheric Pressure. Nanomaterials. 2019; 9(12):1734. https://doi.org/10.3390/nano9121734
Chicago/Turabian StyleChung, Jai Young, Satoshi Kodama, and Hidetoshi Sekiguchi. 2019. "Preparation of a Pd/Al2O3 Catalyst with Microwave-Induced Plasma Jet Irradiation under Atmospheric Pressure" Nanomaterials 9, no. 12: 1734. https://doi.org/10.3390/nano9121734





