A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with a Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TOCNFs
2.3. Synthesis of TOCNF/PAA-PPy Hybrid Hydrogels
2.4. Characterization
3. Results and Discussion
3.1. Design of TOCNF/PAA-PPy Hybrid Hydrogels
3.2. Morphology and Chemical Structure of Hydrogels
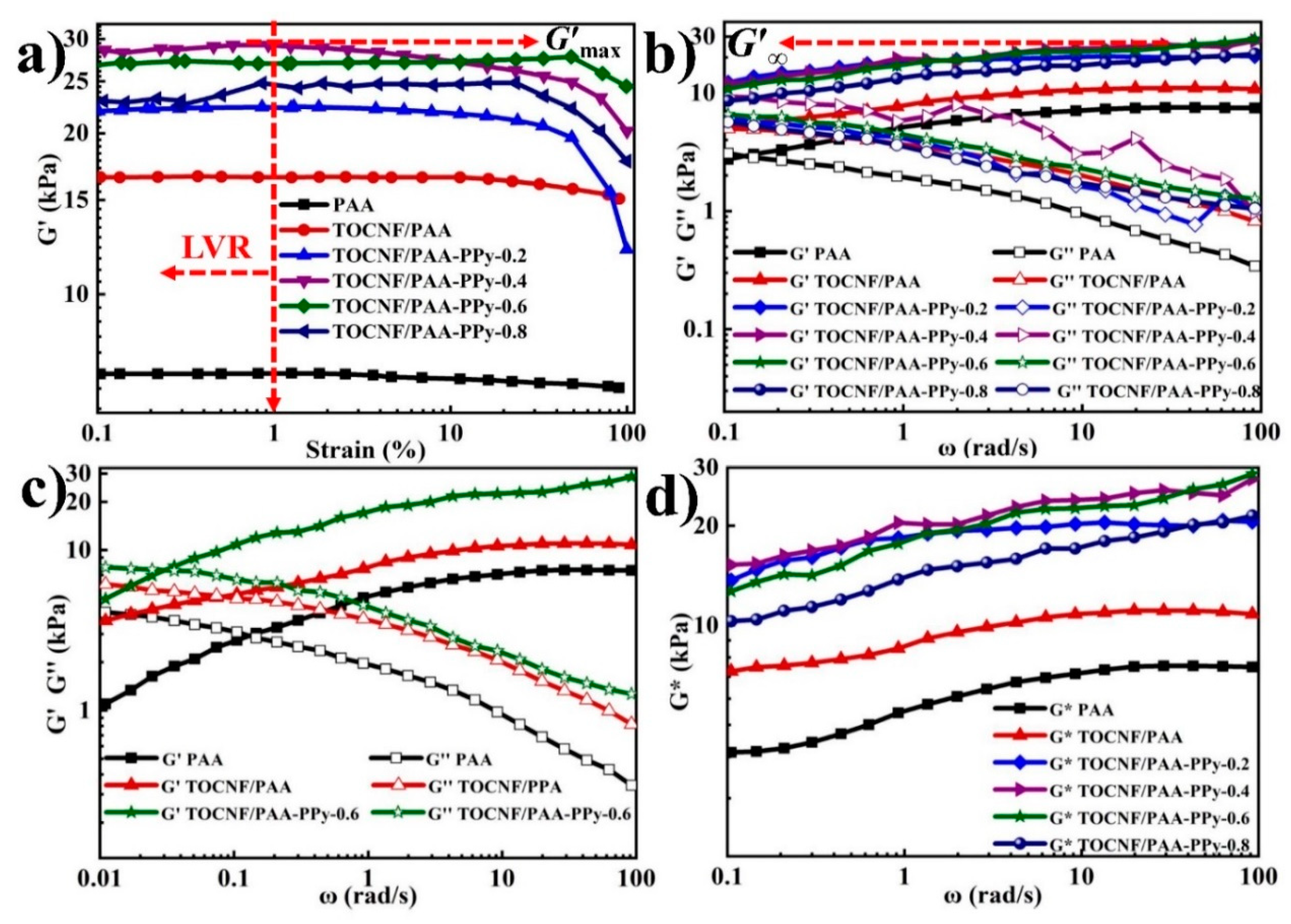
3.3. Dynamic Viscoelasticity of TOCNF/PAA-PPy Hybrid Hydrogels
3.4. Mechanical Performance of Composite Gels
3.5. Self-Healability of TOCNF/PAA-PPy Gels
3.6. Electro-Conductivity of TOCNF/PAA-PPy Hydrogels
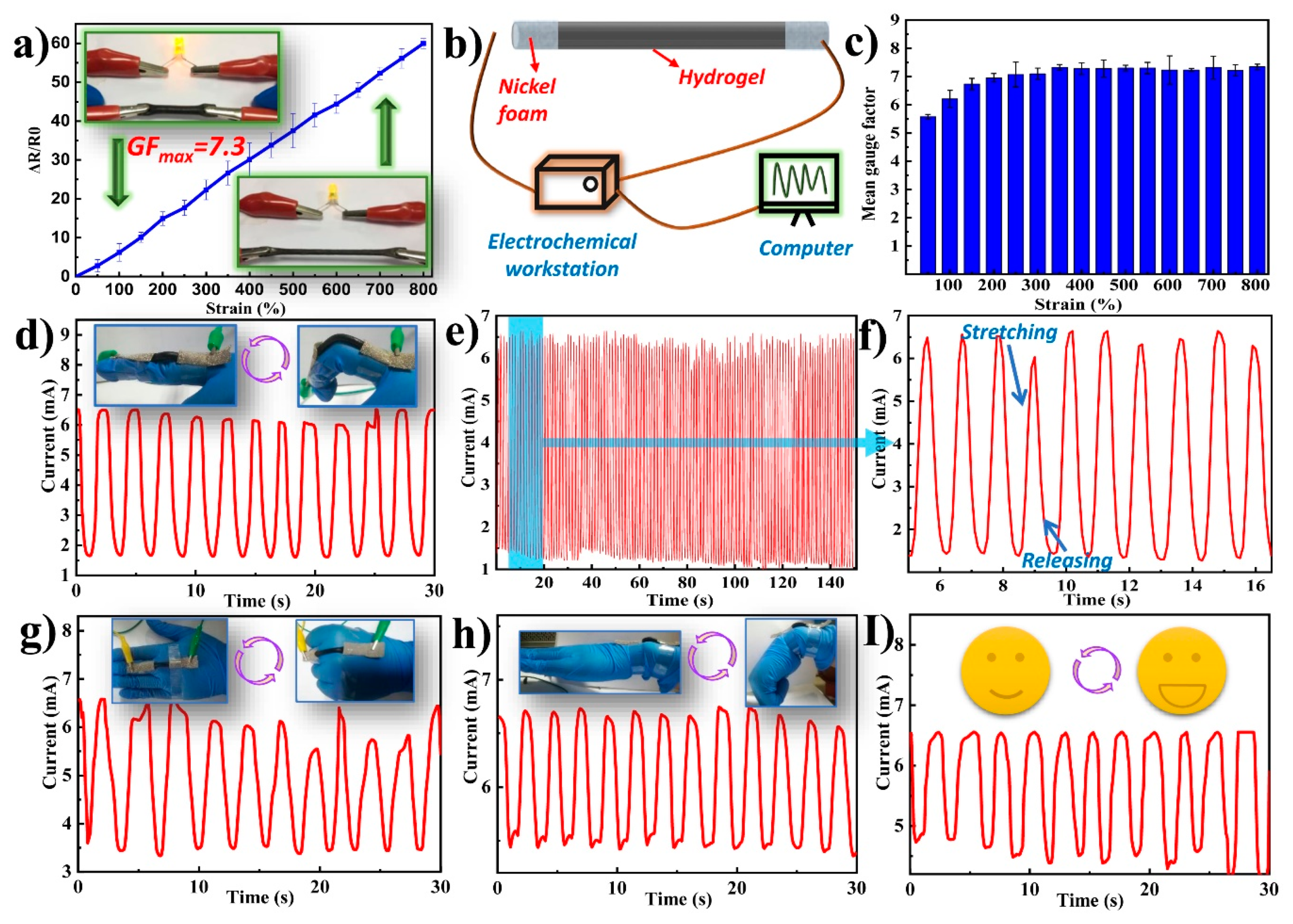
3.7. Sensing Performance Analysis of TOCNF/PAA-PPy Based Strain Sensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Luo, Y.; Liu, J.; Zhang, X.; Lu, C. A well-organized graphene nanostructure for versatile strain-sensing application constructed by a covalently bonded graphene/rubber interface. J. Mater. Chem. C 2018, 6, 2139–2147. [Google Scholar] [CrossRef]
- Han, J.; Lu, K.; Yue, Y.; Mei, C.; Huang, C.; Wu, Q.; Xu, X. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crops Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hussain, I.; Oderinde, O.; Yao, F.; Zhang, J.; Fu, G. A Conductive Self-Healing Double Network Hydrogel with Toughness and Force Sensitivity. Chem. A Eur. J. 2018, 24, 6632–6638. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.; Pei, Z.; Wang, Z.; Xue, Q.; Xie, X.; Zhi, C. A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely stretchable strain sensors based on conductive self-healing dynamic cross-links hydrogels for human-motion detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef]
- Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials 2019, 9, 937. [Google Scholar] [CrossRef]
- Han, J.; Wang, H.; Yue, Y.; Mei, C.; Chen, J.; Huang, C.; Wu, Q.; Xu, X. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Han, J.; Ding, Q.; Mei, C.; Wu, Q.; Yue, Y.; Xu, X. An intrinsically self-healing and biocompatible electroconductive hydrogel based on nanostructured nanocellulose-polyaniline complexes embedded in a viscoelastic polymer network towards flexible conductors and electrodes. Electrochim. Acta 2019, 318, 660–672. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-mediated electroconductive self-healing hydrogels with high strength, plasticity, viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core-Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 44624–44635. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Yao, F.; Oderinde, O.; Fu, G. Hydroxyethyl cellulose-based self-healing hydrogels with enhanced mechanical properties via metal-ligand bond interactions. Eur. Polym. J. 2018, 100, 219–227. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Liu, Q.; Cheng, W.; Wang, X.; Pan, L.; Xu, B.; Xu, H. A self-healable, highly stretchable, and solution processable conductive polymer composite for ultrasensitive strain and pressure sensing. Adv. Funct. Mater. 2018, 28, 1705551. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Ying, S.; Zheng, W.; Li, B.; She, X.; Huang, H.; Li, L.; Huang, Z.; Huang, Y.; Liu, Z.; Yu, X. Facile fabrication of elastic conducting polypyrrole nanotube aerogels. Synth. Met. 2016, 218, 50–55. [Google Scholar] [CrossRef]
- Anjum, S.; Gurave, P.M.; Gupta, B. Calcium ion-induced self-healing pattern of chemically crosslinked poly (acrylic acid) hydrogels. Polym. Int. 2018, 67, 250–257. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-strength, tough, and self-healing nanocomposite physical hydrogels based on the synergistic effects of dynamic hydrogen bond and dual coordination bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Lai, J.; Yan, B.; Liu, H.; Jin, X.; Ma, A.; Zhang, G.; Zhao, W.; Chen, W. Extremely stretchable and electrically conductive hydrogels with dually synergistic networks for wearable strain sensors. J. Mater. Chem. C 2018, 6, 9200–9207. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Lv, W.; Xu, H.; Yang, H.; Yan, W. Synthesis of polypyrrole nano-fibers with hierarchical structure and its adsorption property of Acid Red G from aqueous solution. Synth. Met. 2014, 191, 66–73. [Google Scholar] [CrossRef]
- Yu, H.; Chen, P.; Chen, W.; Liu, Y. Effect of cellulose nanofibers on induced polymerization of aniline and formation of nanostructured conducting composite. Cellulose 2014, 21, 1757–1767. [Google Scholar] [CrossRef]
- Anjum, S.; Gurave, P.; Badiger, M.V.; Torris, A.; Tiwari, N.; Gupta, B. Design and development of trivalent aluminum ions induced self-healing polyacrylic acid novel hydrogels. Polymer 2017, 126, 196–205. [Google Scholar] [CrossRef]
- Fujisawa, S.; Saito, T.; Kimura, S.; Iwata, T.; Isogai, A. Surface engineering of ultrafine cellulose nanofibrils toward polymer nanocomposite materials. Biomacromolecules 2013, 14, 1541–1546. [Google Scholar] [CrossRef]
- Oyama, H.T.; Tang, W.T.; Frank, C.W. Complex formation between poly (acrylic acid) and pyrene-labeled polyethylene glycol in aqueous solution. Macromolecules 1987, 20, 474–480. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 2008, 10, 162–165. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Liu, H.; Du, C.; Yu, H.; Zhou, Z. Thermo-responsive and compression properties of TEMPO-oxidized cellulose nanofiber-modified PNIPAm hydrogels. Carbohydr. Polym. 2016, 147, 201–207. [Google Scholar] [CrossRef]
- Ozkazanc, E. Polypyrrole/copper (II) acetylacetonate composites prepared by in situ chemical oxidative polymerisation. Synth. Met. 2012, 162, 1016–1023. [Google Scholar] [CrossRef]
- Müller, D.; Rambo, C.; Recouvreux, D.; Porto, L.; Barra, G. Chemical in situ polymerization of polypyrrole on bacterial cellulose nanofibers. Synth. Met. 2011, 161, 106–111. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Devan, R.S.; Gang, M.G.; Ma, Y.-R.; Kim, J.H.; Patil, P.S. Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite. Electrochim. Acta 2013, 105, 569–577. [Google Scholar] [CrossRef]
- Xiao, Y.; He, L.; Che, J. An effective approach for the fabrication of reinforced composite hydrogel engineered with SWNTs, polypyrrole and PEGDA hydrogel. J. Mater. Chem. 2012, 22, 8076–8082. [Google Scholar] [CrossRef]
- Alam, A.; Zhang, Y.; Kuan, H.-C.; Lee, S.-H.; Ma, J. Polymer composite hydrogels containing carbon nanomaterials—Morphology and mechanical and functional performance. Prog. Polym. Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
- Han, J.Q.; Lei, T.Z.; Wu, Q.L. High-water-content mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Dynamic rheological properties and hydrogel formation mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Colloids Surf. B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohydr. Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- Li, M.; Zu, M.; Yu, J.; Cheng, H.; Li, Q. Stretchable fiber supercapacitors with high volumetric performance based on buckled MnO2/oxidized carbon nanotube fiber electrodes. Small 2017, 13, 1602994. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide–poly (acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Zhao, Y.; Long, S.; Zheng, J. Dual physically crosslinked double network hydrogels with high toughness and self-healing properties. Soft Matter 2017, 13, 911–920. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, C.-Y.; Qu, D.-H.; Long, Y.-T.; Feringa, B.L.; Tian, H. Exploring a naturally tailored small molecule for stretchable, self-healing, and adhesive supramolecular polymers. Sci. Adv. 2018, 4, eaat8192. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C. A highly stretchable autonomous self-healing elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Cho, M.S.; Choi, J.; Lee, Y. Fabrication of polypyrrole (PPy)/carbon nanotube (CNT) composite electrode on ceramic fabric for supercapacitor applications. Electrochim. Acta 2011, 56, 7460–7466. [Google Scholar] [CrossRef]
- Beattie, D.; Wong, K.H.; Williams, C.; Poole-Warren, L.A.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Honeycomb-structured porous films from polypyrrole-containing block copolymers prepared via RAFT polymerization as a scaffold for cell growth. Biomacromolecules 2006, 7, 1072–1082. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E.; Han, X.; Rubloff, G.W.; Hu, L.; Lee, S.B. Natural cellulose fiber as substrate for supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- He, C.; Yang, C.; Li, Y. Chemical synthesis of coral-like nanowires and nanowire networks of conducting polypyrrole. Synth. Met. 2003, 139, 539–545. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Zhang, P.; Huo, J.; Ericson, F.; Strømme, M.; Nyholm, L. Freestanding nanocellulose-composite fibre reinforced 3D polypyrrole electrodes for energy storage applications. Nanoscale 2014, 6, 13068–13075. [Google Scholar] [CrossRef]
- Ge, S.; Zhao, Z.; Pai, J.; Lee, I.; Ma, J. Highly Sensitive, Wearable, Durable Strain Sensors, and Stretchable Conductors Using Graphene/Silicon Rubber Composites. Adv. Funct. Mater. 2016, 26, 7614–7625. [Google Scholar]
- Miyamoto, A.; Lee, S.; Cooray, N.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Interfaces, Ultrastretchable and Self-Healing Double-Network Hydrogel for 3D Printing and Strain Sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef]
- Frutiger, A.; Muth, J.T.; Vogt, D.M.; Mengü, Y.I.; Campo, A.; Valentine, A.D.; Walsh, C.J.; Lewis, J.A. Capacitive Soft Strain Sensors via Multicore-Shell Fiber Printing. Adv. Mater. 2015, 27, 2440–2446. [Google Scholar] [CrossRef]
- Sengupta, D.; Pei, Y.; Kottapalli, A.G. Ultralightweight and 3D Squeezable Graphene-Polydimethylsiloxane Composite Foams as Piezoresistive Sensors. ACS Appl. Mater. Interfaces 2019, 11, 35201–35211. [Google Scholar] [CrossRef]





| AA (g) | H2O (mL) | MBA/AA (w/w) | FeCl3/AA (w/w) | APS (mg) | PA (g) | Py (mol L−1) | TOCNFs/AA (w/w) | Hydrogel Designation |
|---|---|---|---|---|---|---|---|---|
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0 | 0 | PAA |
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0 | 2 | TOCNF/PAA |
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0.2 | 2 | TOCNF/PAA-PPy-0.2 |
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0.4 | 2 | TOCNF/PAA-PPy-0.4 |
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0.6 | 2 | TOCNF/PAA-PPy-0.6 |
| 6 | 24 | 0.5 | 0.9 | 12 | 2 | 0.8 | 2 | TOCNF/PAA-PPy-0.8 |
| Parameter | Gel-0.2 | Gel-0.4 | Gel-0.6 | Gel-0.8 | TOCNF/PAA | PAA |
|---|---|---|---|---|---|---|
| Critical strains, γc (%) | 25.1 | 21.7 | 16.2 | 11.5 | 33.7 | n.a. |
| G’max (kPa) | 21.1 | 28.9 | 27.1 | 24.4 | 16.6 | 7.2 |
| G’∞ (kPa) | 20.6 | 27.7 | 28.7 | 21.4 | 7.6 | 11.1 |
| Sample | σt (MPa) | εt (%) | σe (MPa) at εe = 60% | Wc (%) | ρ (g cm−3) |
|---|---|---|---|---|---|
| PAA | 0.25 ± 0.05 | 795 ± 53 | 0.14 ± 0.02 | 81.9 ± 0.5 | 1.1 ± 0.1 |
| TOCNF/PAA | 0.36 ± 0.03 | 1069 ± 26 | 0.23 ± 0.03 | 82.0 ± 0.6 | 1.1 ± 0.2 |
| TOCNF/PAA-PPy-0.2 | 0.41 ± 0.07 | 644 ± 52 | 0.46 ± 0.04 | 82.0 ± 0.8 | 1.2 ± 0.1 |
| TOCNF/PAA-PPy-0.4 | 0.60 ± 0.10 | 588 ± 21 | 1.65 ± 0.12 | 81.4 ± 0.7 | 1.2 ± 0.4 |
| TOCNF/PAA-PPy-0.6 | 0.55 ± 0.05 | 889 ± 46 | 0.67 ± 0.08 | 82.2 ± 0.4 | 1.3 ± 0.2 |
| TOCNF/PAA-PPy-0.8 | 0.52 ± 0.06 | 719 ± 33 | 0.24 ± 0.03 | 82.1 ± 0.6 | 1.2 ± 0.1 |
| Sample | f1 After 2 h Healing | f1 After 4 h Healing | f1 After 6 h Healing |
|---|---|---|---|
| TOCNF/PAA | 28.9% | 64.6% | 73.5% |
| TOCNF/PAA-PPy-0.2 | 71.3% | 84.1% | 95.3% |
| TOCNF/PAA-PPy-0.4 | 81.4% | 91.5% | 97.5% |
| TOCNF/PAA-PPy-0.6 | 73.0% | 87.9% | 98.3% |
| TOCNF/PAA-PPy-0.8 | 58.4% | 87.0% | 96.7% |
| Sample | σR (S m−1) | σ’R (S m−1) | ƒ2 (%) |
|---|---|---|---|
| PAA | 2.3 ± 0.1 | 2.3 ± 0.1 | 99.5 |
| TOCNF/PAA | 2.3 ± 0.1 | 2.3 ± 0.2 | 99.5 |
| TOCNF/PAA-PPy-0.2 | 2.4 ± 0.2 | 2.4 ± 0.1 | 99.1 |
| TOCNF/PAA-PPy-0.4 | 3.2 ± 0.1 | 3.2 ± 0.2 | 98.7 |
| TOCNF/PAA-PPy-0.6 | 3.9 ± 0.2 | 3.8 ± 0.1 | 99.4 |
| TOCNF/PAA-PPy-0.8 | 4.2 ± 0.1 | 4.2 ± 0.3 | 99.0 |
| Material | Gauge Factor | Linearity |
|---|---|---|
| PAAerGO nanocomposite [8] | 0.31–1.32 | nonlinear |
| PAA-PANI [16] | 0.60–1.05 | two linear regions |
| PANI-PAA [23] | 4.7–11.6 | two linear regions |
| CNCs, PVA and PVP [4] | ~0.478 | nonlinear |
| κ-carrageenan/PAAm DN [53] | ~0.63 | nonlinear |
| PVA-PAA-MNPs [54] | ~0.06 | linear |
| Fibers-silicone [55] | ~0.348 | nonlinear |
| This work | ~7.3 | two linear regions |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lu, K.; Song, Y.; Han, J.; Yue, Y.; Biswas, S.K.; Wu, Q.; Xiao, H. A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with a Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection. Nanomaterials 2019, 9, 1737. https://doi.org/10.3390/nano9121737
Chen Y, Lu K, Song Y, Han J, Yue Y, Biswas SK, Wu Q, Xiao H. A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with a Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection. Nanomaterials. 2019; 9(12):1737. https://doi.org/10.3390/nano9121737
Chicago/Turabian StyleChen, Yuanyuan, Kaiyue Lu, Yuhan Song, Jingquan Han, Yiying Yue, Subir Kumar Biswas, Qinglin Wu, and Huining Xiao. 2019. "A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with a Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection" Nanomaterials 9, no. 12: 1737. https://doi.org/10.3390/nano9121737
APA StyleChen, Y., Lu, K., Song, Y., Han, J., Yue, Y., Biswas, S. K., Wu, Q., & Xiao, H. (2019). A Skin-Inspired Stretchable, Self-Healing and Electro-Conductive Hydrogel with a Synergistic Triple Network for Wearable Strain Sensors Applied in Human-Motion Detection. Nanomaterials, 9(12), 1737. https://doi.org/10.3390/nano9121737







