Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry
Abstract
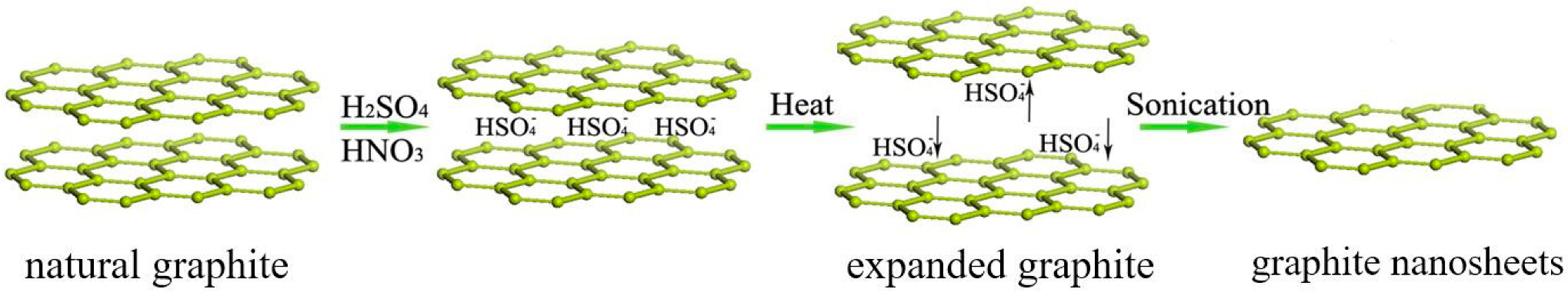
:1. Introduction
2. Experimental Section
2.1. Materials
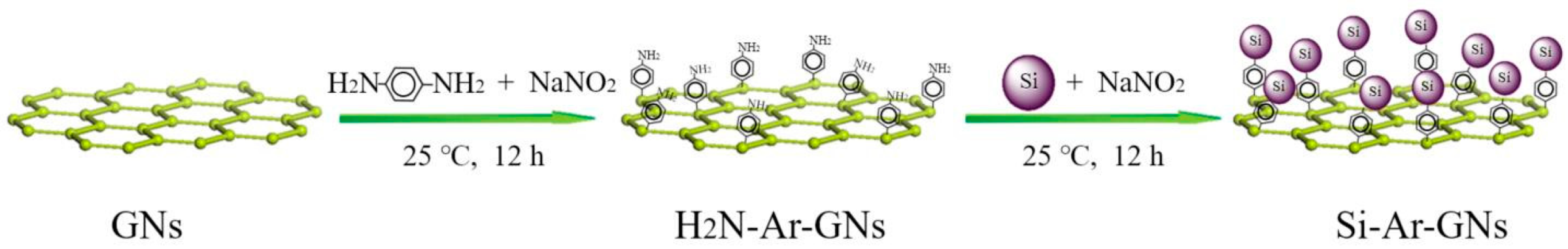
2.2. Synthesis of Si–Ar–GNs Composite
2.3. Characterization
2.4. Electrochemical Characterization
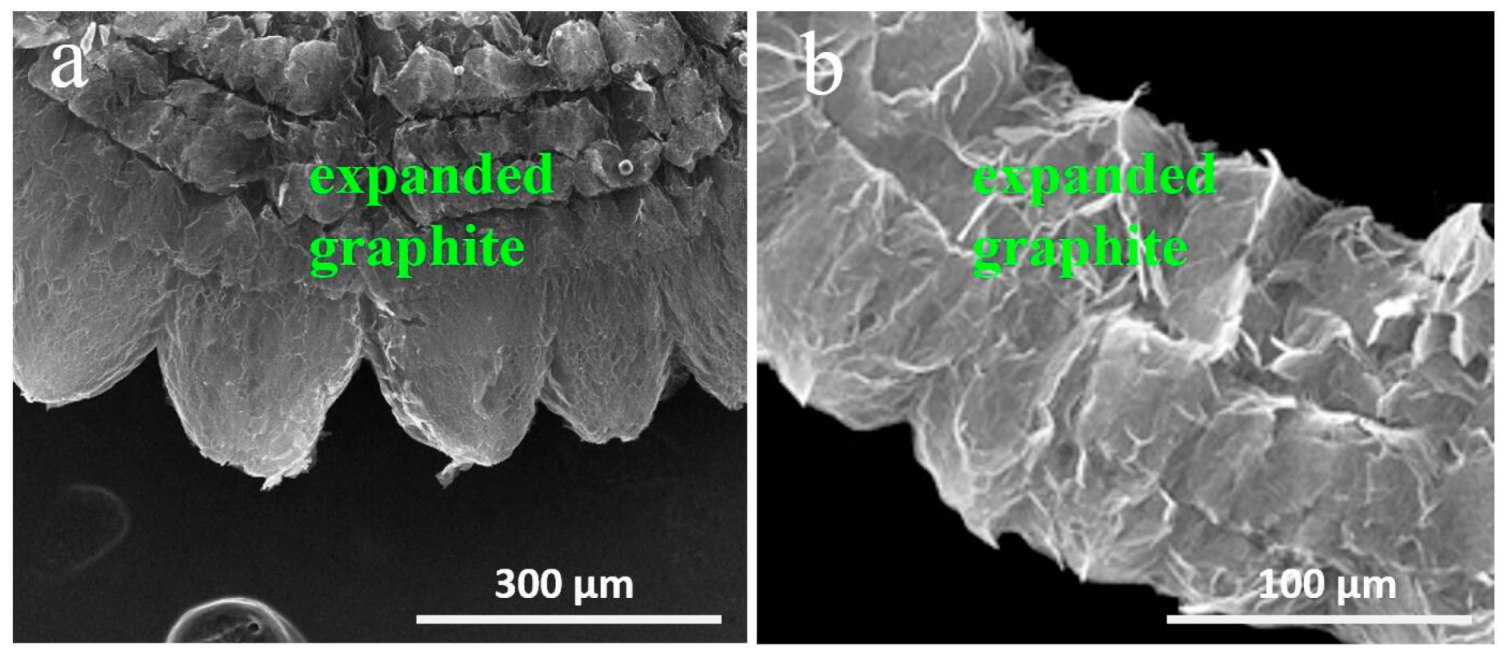
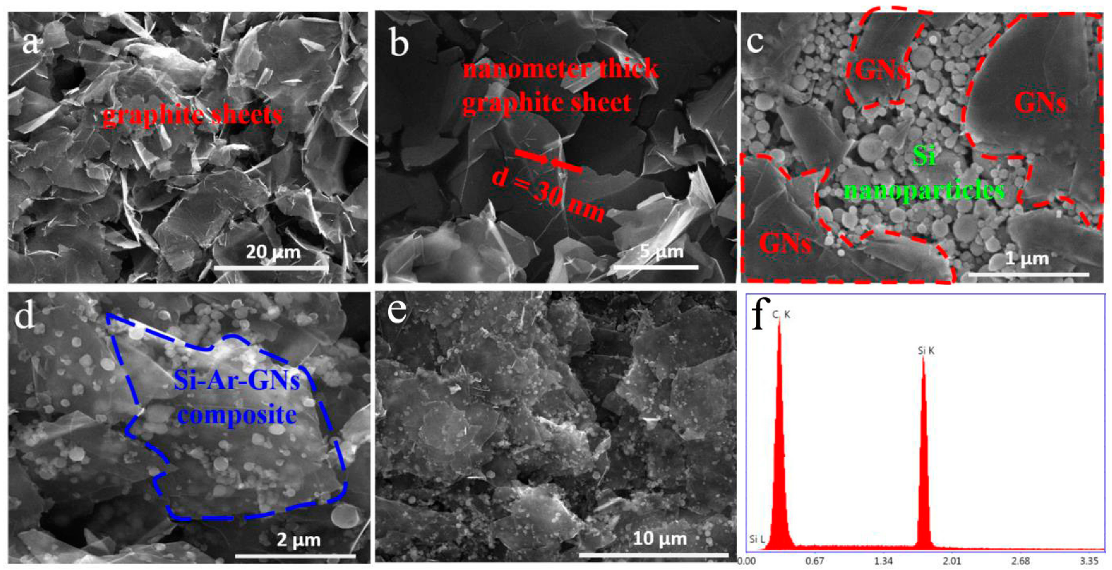
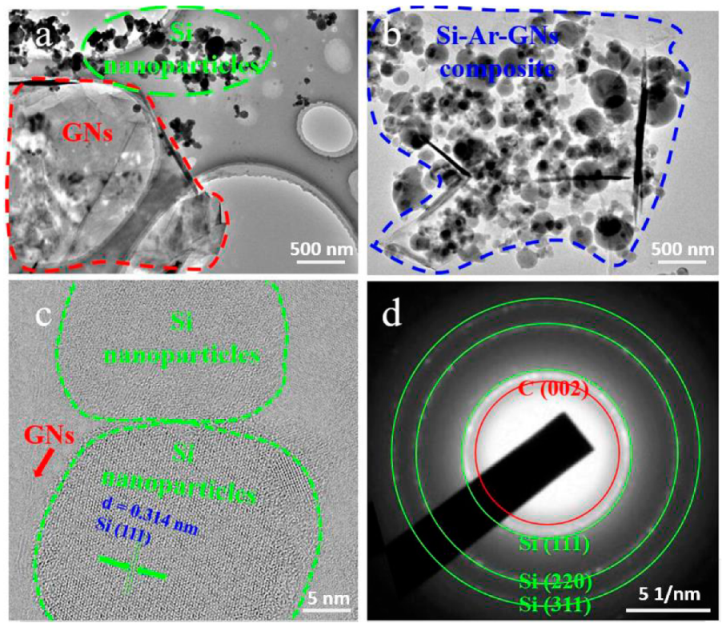
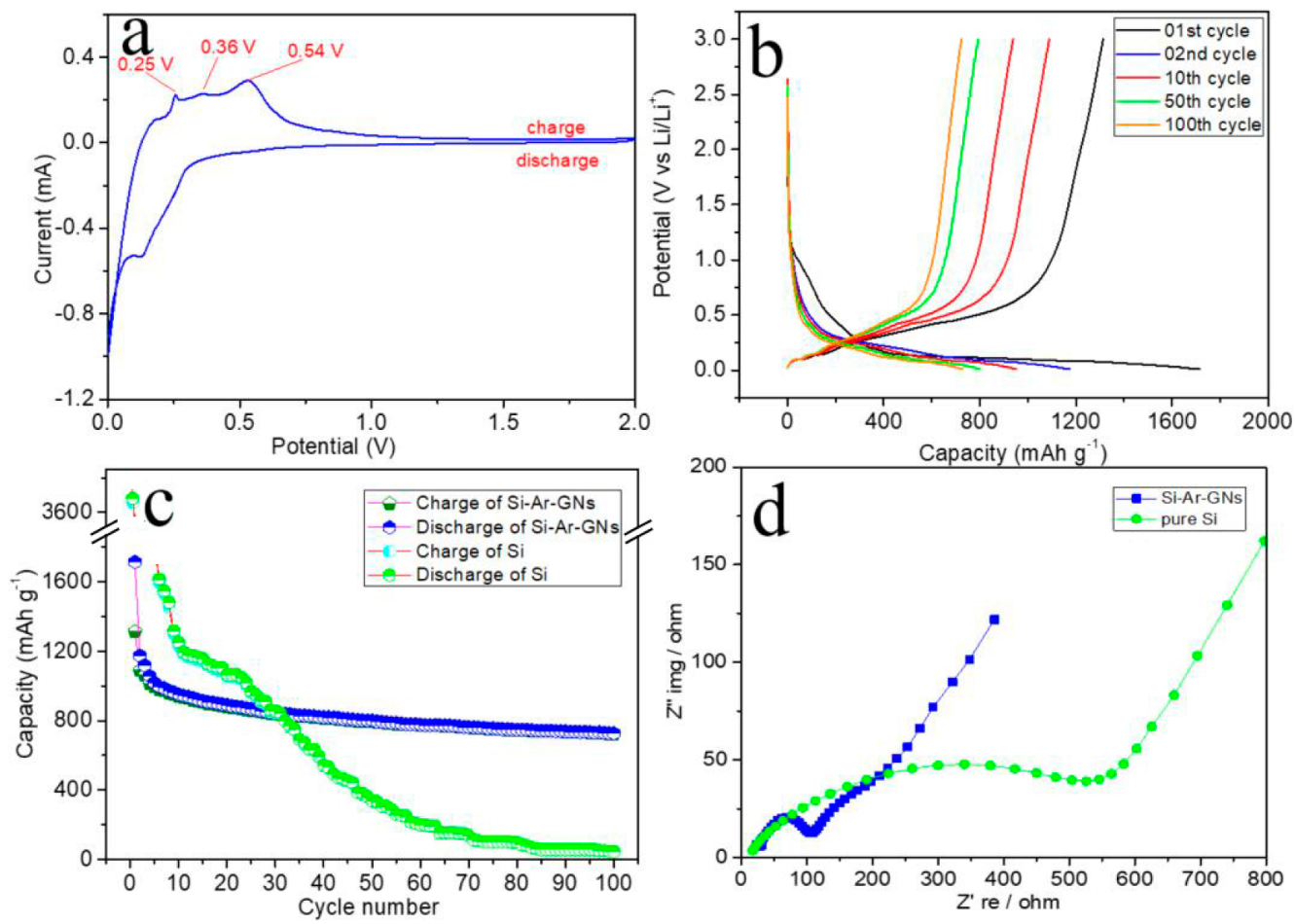
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Feng, Y.; Zhang, T.; Chen, Q.; Chi, Q.; Liu, L.; Li, G.; Cui, Y.; Wang, X.; et al. Excellent energy storage performance and thermal property of polymer-based composite induced by multifunctional one-dimensional nanofibers oriented in-plane direction. Nano Energy 2019, 56, 138–150. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y. Lithium-ion batteries. In a look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Yuan, L.X.; Wang, Z.H.; Zhang, W.X.; Hu, X.L.; Chen, J.T.; Huang, Y.H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2011, 4, 269–284. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Wang, J.; Li, N.; Wu, M.; Zhang, Q.; Du, Y. Interplanar space-controllable carboxylate pillared metal organic framework ultrathin nanosheet for superhigh capacity rechargeable alkaline battery. Nano Energy 2019, 62, 876–882. [Google Scholar] [CrossRef]
- Vernardou, D.; Kazas, A.; Apostolopoulou, M.; Katsarakis, N.; Koudoumas, E. Cationic effect on the electrochemical characteristics of the hydrothermally grown manganese dioxide. J. Electron. Mater. 2017, 46, 2232–2240. [Google Scholar] [CrossRef]
- Wu, G.; Jia, Z.; Cheng, Y.; Zhang, H.; Zhou, X.; Wu, H. Easy synthesis of multi-shelled ZnO hollow spheres and their conversion into hedgehog-like ZnO hollow spheres with superior rate performance for lithium ion batteries. Appl. Surf. Sci. 2019, 464, 472–478. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Zhang, Y.; Zhang, Y.; Wu, Y.; Tian, M.; Chen, P.; Robert, T.; Ma, Y.; Wu, T.; et al. A safe and fast-charging lithium-ion battery anode using MXene supported Li3VO4. J. Mater. Chem. A 2019, 7, 11250–11256. [Google Scholar] [CrossRef]
- Wu, G.; Wu, H.; Wang, K.; Zheng, C.; Wang, Y.; Feng, A. Facile synthesis and application of multi-shelled SnO2 hollow spheres in lithium ion battery. RSC Adv. 2016, 6, 58069–58076. [Google Scholar] [CrossRef]
- Wu, X.; Huang, B.; Wang, Q.; Wang, Y. High energy density of two-dimensional MXene/NiCo-LDHs interstratification assembly electrode: Understanding the role of interlayer ions and hydration. Chem. Eng. J. 2020, 380, 122456. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zheng, C.; Zhu, J.; Wu, G.; Li, X. Multi-shelled NiO hollow spheres: Easy hydrothermal synthesis and Lithium storage performances. J. Alloys Compd. 2016, 685, 8–14. [Google Scholar] [CrossRef]
- Wang, K.; Tan, Y.; Li, P.; Xue, B.; Sun, J. Facile synthesis of double layer constrained micron-sized porous Si/SiO2/C composites for lithium ion battery anodes. ACS Appl. Mater. Interfaces 2019, 11, 37732–37740. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Wang, Z.; Gong, X.; Wang, Y. A new design for Si wears double jackets used as a high-performance lithium-ion battery anode. Chem. Eng. J. 2019, 370, 565–572. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Ren, J.; Wu, Y.; Yu, N.; Feng, A.; Zhuang, Z.; Jia, Z.; Wu, G. Sandwich-like Si/SiC/nanographite sheet as high performance anode for lithium-ion batteries. Dalton Trans. 2019, 48, 17683–17690. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhu, X.; Li, F.; Lai, F.; Wu, Y.; Miao, Y.E.; Liu, T. Silicon@nitrogen-doped porous carbon fiber composite anodes synthesized by an in-situ reaction collection strategy for high-performance lithium-ion batteries. Appl. Surf. Sci. 2019, 475, 211–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Fu, L.; Meng, J.; Yu, N.; Wang, J.; Wu, Y. Si/C composites as negative electrode for high energy lithium ion batteries. Chin. J. Chem. 2017, 35, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Sui, X.; Yang, H.; Ren, R.; Wu, Y.; Guo, X.; Chen, J. HF-free synthesis of Si/C yolk/shell anodes for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 2593–2599. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, Y.; Yang, J.; Ding, J.; Zhang, J.; Hu, T.; Tang, J. Si nanoflake-assembled blocks towards high initial coulombic efficiency anodes for lithium-ion batteries. Chem. Commun. 2018, 54, 12214–12217. [Google Scholar] [CrossRef]
- Su, W.; Liang, Y.; Zuo, Y.; Tang, Y. A facile in situ synthesis of SiC&Si@CNT composite 3D frameworks as an anode material for lithium-ion batteries. Dalton Trans. 2019, 48, 12964–12973. [Google Scholar]
- Zhang, Y.; Hu, K.; Zhou, Y.; Xia, Y.; Yu, N.; Wu, G.; Zhu, Y.; Wu, Y.; Huang, H. A facile, one-step synthesis of silicon/silicon carbide/carbon nanotube nanocomposite as a cycling-stable anode for lithium ion batteries. Nanomaterials 2019, 9, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jeong, Y.K.; Wang, Y.; Lee, H.; Choi, J.W. A “Sticky” Mucin-Inspired DNA-Polysaccharide Binder for Silicon and Silicon-Graphite Blended Anodes in Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1707594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Li, Y.; Li, B.; Li, Z.; Niu, C. Preparation of nanographite sheets supported Si nanoparticles by in situ reduction of fumed SiO2 with magnesium for lithium ion battery. J. Power Sources 2015, 281, 425–431. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C. PMMA/graphite nanosheets composite and its conducting properties. Eur. Polym. J. 2003, 39, 2329–2335. [Google Scholar] [CrossRef]
- Chen, X.; Shi, T.; Zhong, K.; Wu, G.; Lu, Y. Capacitive behavior of MoS2 decorated with FeS2@carbon nanospheres. Chem. Eng. J. 2020, 379, 122240. [Google Scholar]
- Zhang, Y.; Qi, S.; Wu, X.; Duan, G. Electrically conductive adhesive based on acrylate resin filled with silver plating graphite nanosheet. Synth. Met. 2011, 161, 516–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, S.; Zhang, F.; Yang, Y.; Duan, G. Preparation and magnetic properties of polymer magnetic composites based on acrylate resin filled with nickel plating graphite nanosheets. Appl. Surf. Sci. 2011, 258, 732–737. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Zhu, Q.; Pan, Q. Covalent binding of Si nanoparticles to graphene sheets and its influence on lithium storage properties of Si negative electrode. J. Mater. Chem. 2012, 22, 3420–3425. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J. Mater. Chem. 2010, 20, 8467–8469. [Google Scholar] [CrossRef]
- Sun, G.; Li, X.; Qu, Y.; Wang, X.; Yan, H.; Zhang, Y. Preparation and characterization of graphite nanosheets from detonation technique. Mater. Lett. 2008, 62, 703–706. [Google Scholar] [CrossRef]
- Li, Y.; Tang, L.; Li, J. Preparation and electrochemical performance for methanol oxidation of Pt/graphene nanocomposites. Electrochem. Commun. 2009, 11, 846–849. [Google Scholar] [CrossRef]
- Xiao, W.; Jin, X.; & Chen, G.Z. Up-scalable and controllable electrolytic production of photo-responsive nanostructured silicon. J. Mater. Chem. A 2013, 1, 10243–10250. [Google Scholar] [CrossRef]
- Du, C.; Chen, M.; Wang, L.; Yin, G. Covalently-functionalizing synthesis of Si@C core-shell nanocomposites as high-capacity anode materials for lithium-ion batteries. J. Mater. Chem. 2011, 21, 15692–15697. [Google Scholar] [CrossRef]
- Zeng, X.B.; Liao, X.B.; Wang, B.; Dai, S.T.; Xu, Y.Y.; Xiang, X.B.; Hu, Z.H.; Diao, H.W.; Kong, G.L. Optical properties of boron-doped Si nanowires. J. Cryst. Growth 2004, 265, 94–98. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, L.D.; Meng, G.W.; Li, G.H.; Jin-Phillipp, N.Y.; Phillipp, F. Synthesis of ordered single crystal silicon nanowire arrays. Adv. Mater. 2001, 13, 1238–1241. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, Y.J.; Rajendran, S.; Song, M.S.; Kim, H.S.; Lee, J.Y. Electrochemical properties of Si/Ni alloy-graphite composite as an anode material for Li-ion batteries. Electrochim. Acta 2005, 50, 5561–5567. [Google Scholar] [CrossRef]
- Fan, X.; Zou, L.; Zheng, Y.P.; Kang, F.Y.; Shen, W.C. Electrospinning preparation of nanosilicon/disordered carbon composite as anode materials in li-ion battery. Electrochem. Solid-State Lett. 2009, 12, A199–A201. [Google Scholar] [CrossRef]
- Ren, Y.; Ding, J.; Yuan, N.; Jia, S.; Qu, M.; Yu, Z. Preparation and characterization of silicon monoxide/graphite/carbon nanotubes composite as anode for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 1453–1460. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Evaluation of Si/carbon composite nanofiber-based insertion anodes for new-generation rechargeable lithium-ion batteries. Energy Environ. Sci. 2010, 3, 124–129. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Du, C.; Ren, Y.; Mu, T.; Zuo, P.; Gao, Y. Polyaniline-encapsulated silicon on three-dimensional carbon nanotubes foam with enhanced electrochemical performance for lithium-ion batteries. J. Power Sources 2018, 381, 156–163. [Google Scholar] [CrossRef]
- Tao, H.; Xiong, L.; Zhu, S.; Yang, X.; Zhang, L. Flexible binder-free reduced graphene oxide wrapped Si/carbon fibers paper anode for high-performance lithium ion batteries. Int. J. Hydrogen Energy 2016, 41, 21268–21277. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ren, J.; Xu, T.; Feng, A.; Hu, K.; Yu, N.; Xia, Y.; Zhu, Y.; Huang, Z.; Wu, G. Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry. Nanomaterials 2019, 9, 1741. https://doi.org/10.3390/nano9121741
Zhang Y, Ren J, Xu T, Feng A, Hu K, Yu N, Xia Y, Zhu Y, Huang Z, Wu G. Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry. Nanomaterials. 2019; 9(12):1741. https://doi.org/10.3390/nano9121741
Chicago/Turabian StyleZhang, Yi, Jinghui Ren, Tao Xu, Ailing Feng, Kai Hu, Nengfei Yu, Yingbin Xia, Yusong Zhu, Zhengyong Huang, and Guanglei Wu. 2019. "Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry" Nanomaterials 9, no. 12: 1741. https://doi.org/10.3390/nano9121741
APA StyleZhang, Y., Ren, J., Xu, T., Feng, A., Hu, K., Yu, N., Xia, Y., Zhu, Y., Huang, Z., & Wu, G. (2019). Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry. Nanomaterials, 9(12), 1741. https://doi.org/10.3390/nano9121741






