Eugenol Polysiloxane-Polycarbonate/Graphene Nanocomposite: Enhanced in Thermostability and Barrier Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Si-PC/GR Nanocomposite
2.3. Chemical Structure of Si-PC/GR Nanocomposite
2.4. Morphology of Si-PC/GR Nanocomposite
2.5. TEM Characterization of Graphene
2.6. Thermostability
2.7. Mechanical Properties
2.8. Oxygen Barrier Property
2.9. Hydrophobicity
3. Results and Discussions
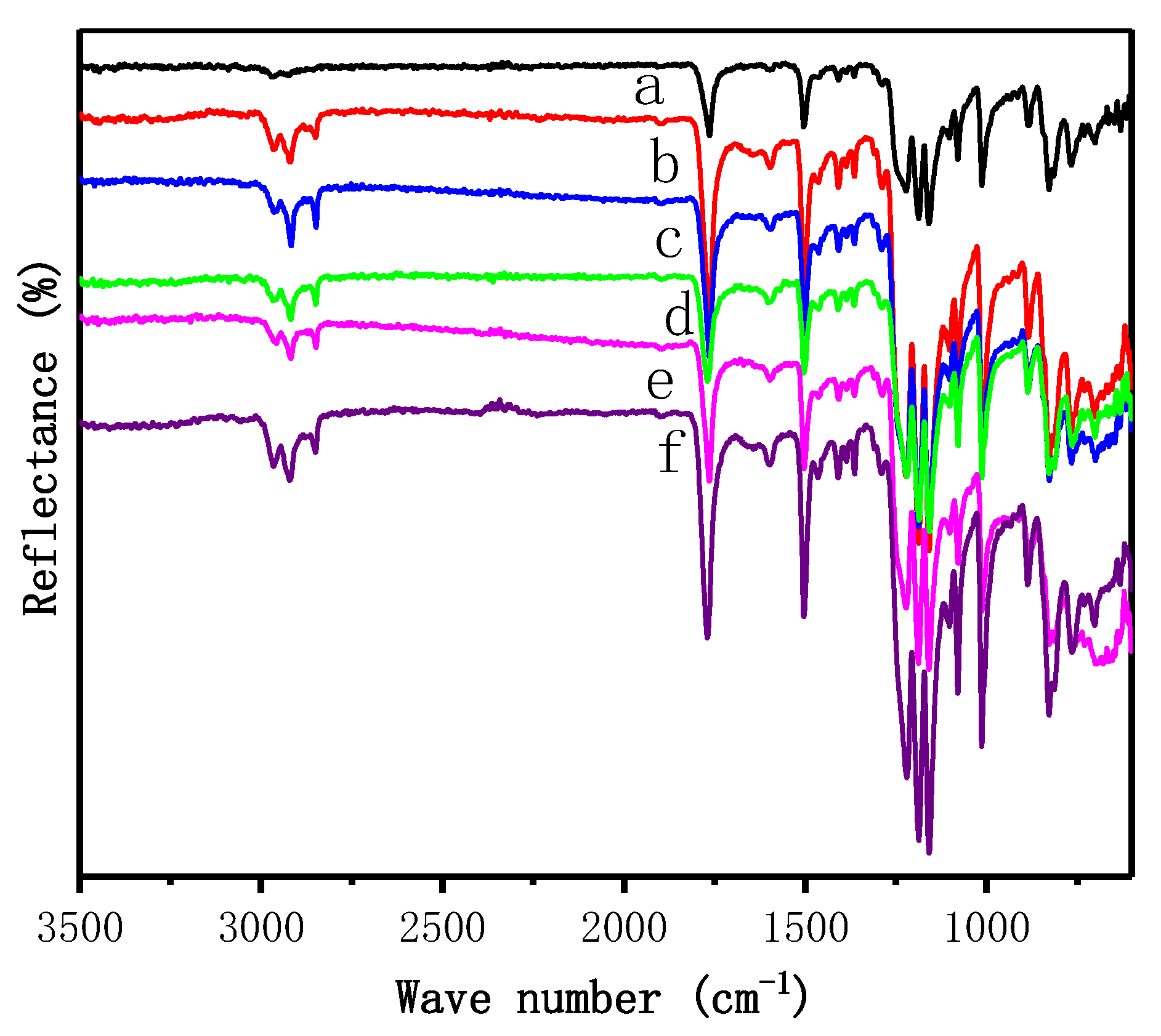
3.1. Chemical Structures of Si-PC/GR Nanocomposites
3.2. Morphology Analysis
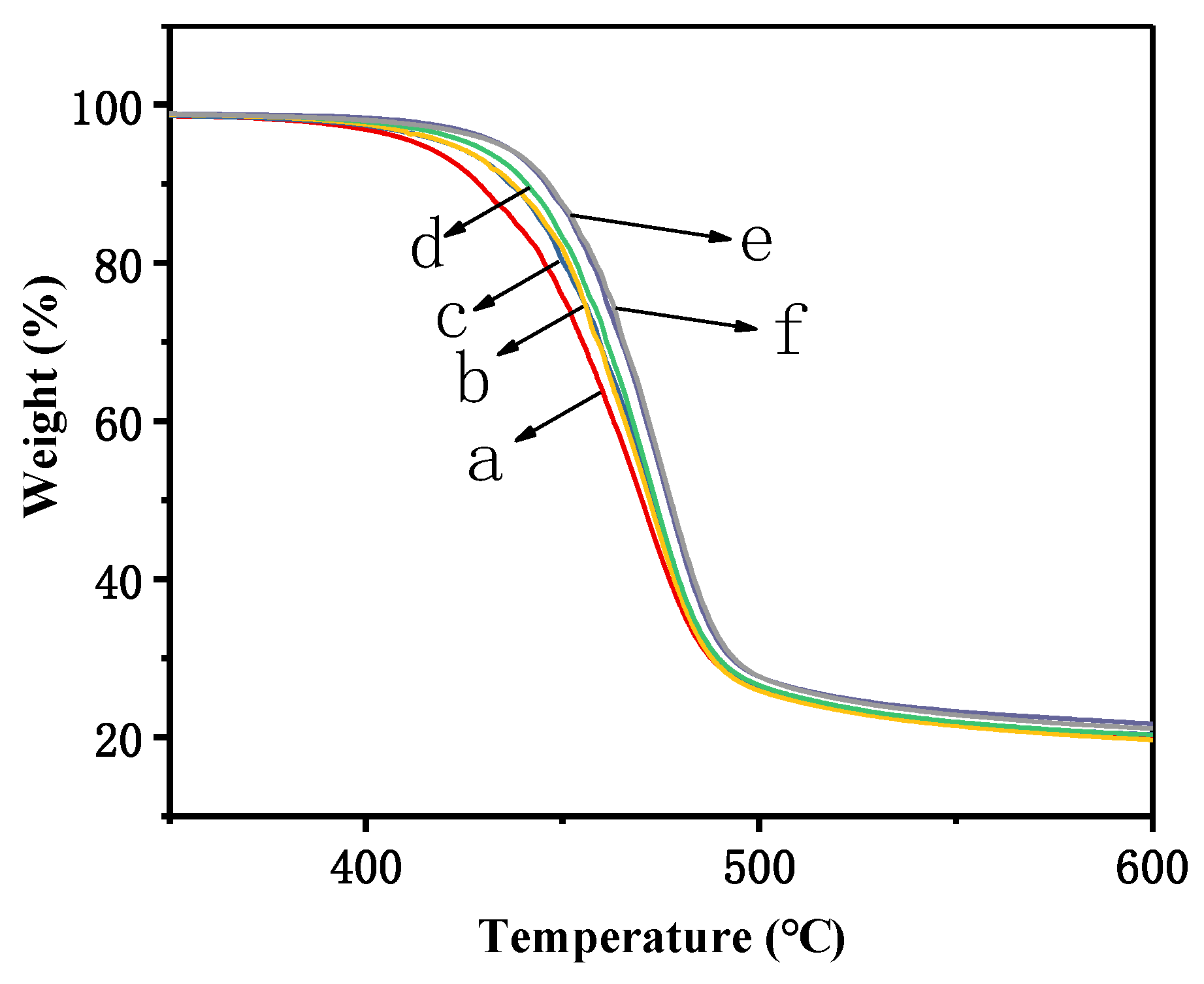
3.3. Thermostability
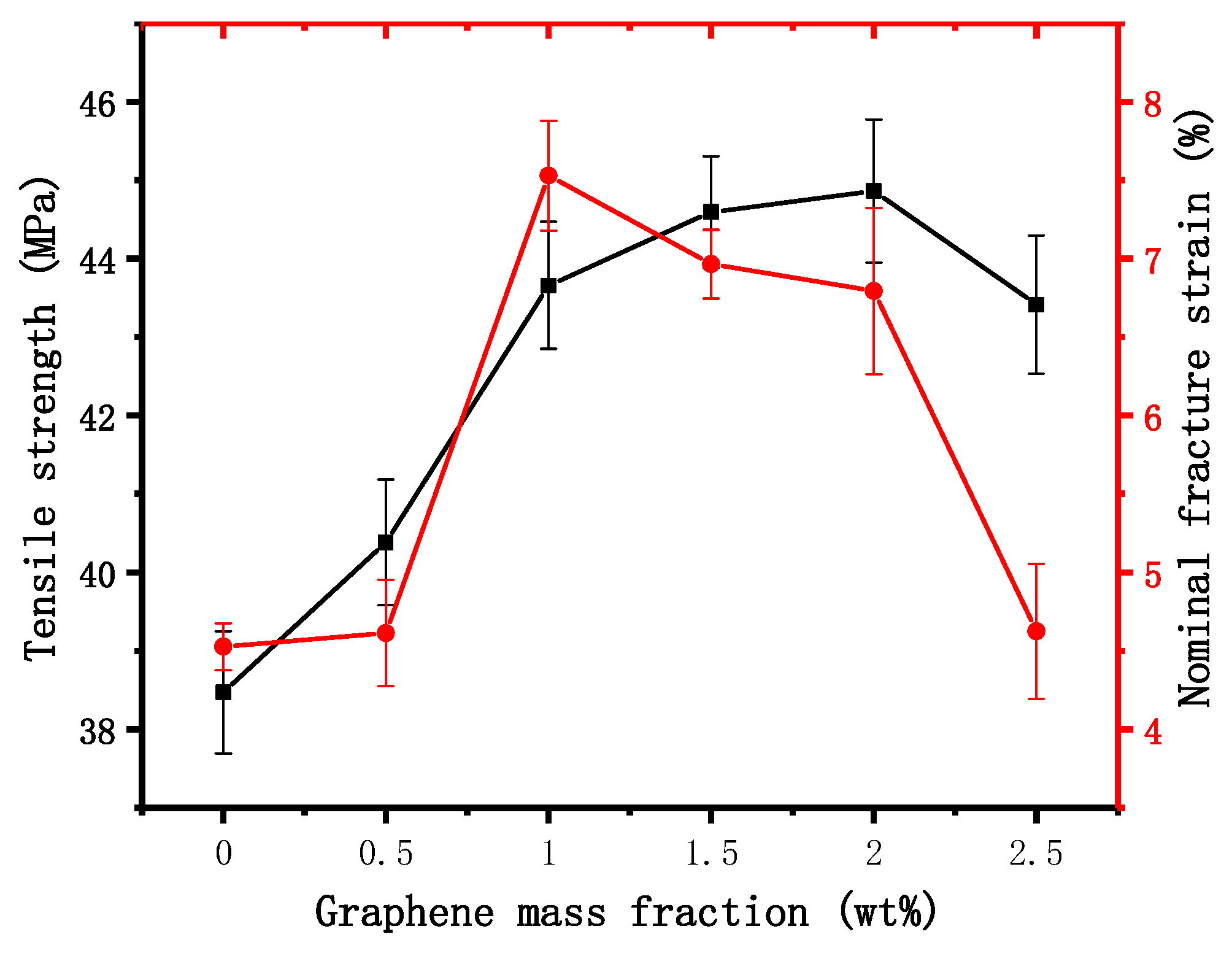
3.4. Mechanical Properties
3.5. Oxygen Barrier Property
3.6. Hydrophobicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potschke, P.; Fornes, T.D.; Paul, D.R. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- Sasaki, H.; Hamanaka, I.; Takahashi, Y.; Kawaguchi, T. Effect of reinforcement on the flexural properties of injection-molded thermoplastic denture base resins. J. Prosthodont. 2015, 26, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Taraghi, I.; Fereidoon, A.; Paszkiewicz, S.; Roslaniec, Z. Electrically conductive polycarbonate/ethylene-propylene copolymer/multi-walled carbon nanotubes nanocomposites with improved mechanical properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Roland, K.; Robert, M.; Michael, F.; Schierle, T. Use of Eugenol Polyethers and Eugenol Polyethers Siloxanes as Wetting Agents. U.S. Patent 9993766B2, 12 June 2018. [Google Scholar]
- Hagenaars, A.C.C.; Bailly, A.S.; Wolf, B.A. Preparative fractionation and characterization of polycarbonate/eugenol-siloxane copolymers. Polymer 2002, 43, 2663–2669. [Google Scholar] [CrossRef]
- Pang, X.Y.; Ge, X.; Ji, J.Y.; Liang, W.; Chen, X.; Ge, J. Facile route for bio-phenol siloxane synthesis via heterogeneous catalytic method and its autonomic antibacterial property. Polymers 2018, 10, 1151. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased epoxy resin derived from eugenol with excellent integrated performances and high renewable carbon content. Polym. Int. 2018. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis of eugenol-based polybenzoxazine-POSS nanocomposites for low dielectric applications. Polym. Compos. 2014, 36, 1973–1982. [Google Scholar] [CrossRef]
- Mangeon, C.; Modjinou, T.; Anda, A.R.D.; Thevenieau, F.; Renard, E.; Langlois, V. Renewable semi-Interpenetrating polymer networks based on vegetable oils used as plasticized systems of poly(3-hydroxyalkanoate)s. ACS Sustain. Chem. Eng. 2018, 6, 5034–5042. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.D.; Zhang, W.H.; Jiang, Q.Y.; Mu, J.X.; Jiang, Z.H. Synthesis and properties of poly(aryl ether sulfone)s incorporating cage and linear organosiloxane in the backbones. Polymer 2015, 62, 77–85. [Google Scholar] [CrossRef]
- Mollah, M.S.I.; Kwon, Y.D.; Islam, M.M.; Seo, D.; Jang, H.; Lim, Y.; Lee, D.; Kim, W. Synthesis and characterization of polycarbonates containing terminal and chain interior siloxane. Polym. Bull. 2012, 68, 1551–1564. [Google Scholar] [CrossRef]
- Pang, X.Y.; Ge, X.; Ji, J.Y.; Liang, W.; Liu, R.; Chen, X.; Yin, G.; Ge, J. Improving oxygen permeability and thermostability of polycarbonate via copolymerization modification with bio-phenol polysiloxane. Polymers 2019, 11, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.Y.; Liang, W.J.; Ge, X.; Ji, J.; Li, Z.; Chen, X.; He, M.; Yin, G.; Ge, J. Preparation and characterizations of SiCw/SEBS-g-MAH composite film. Polym. Polym. Compos. 2019, 27, 383–388. [Google Scholar] [CrossRef]
- Heidari, B.; Ansari, M.; Hoseinabadi, A.; Jiriaee, H.; Heidary, F. The effect of ZnO, Fe3O4, and graphene oxide nanostructures on the microwave absorbing properties of polystyrene composites. J. Mater. Sci. Mater. Electron. 2017, 28, 1028–1037. [Google Scholar] [CrossRef]
- Ren, Z.H.; Jin, P.; Cao, X.M.; Zheng, Y.; Zhang, J.S. Mechanical properties and slurry erosion resistance of SiC ceramic foam/epoxy co-continuous phase composite. Compos. Sci. Technol. 2015, 107, 129–136. [Google Scholar] [CrossRef]
- Yoonessi, M.; Lebrón-Colón, M.; Scheiman, D.; Meador, M.A. Carbon nanotube epoxy nanocomposites: The effects of interfacial modifications on the dynamic mechanical properties of the nanocomposites. ACS Appl. Mater. Interface 2014, 6, 16621–16630. [Google Scholar] [CrossRef]
- Yoonessi, M.; Gaier, J.; Sahimi, M.; Daulton, T.L.; Kaner, R.B.; Meador, M.A. Fabrication of graphene-polyimide nanocomposites with superior electrical conductivity. ACS Appl. Mater. Interface 2017, 9, 43230–43238. [Google Scholar] [CrossRef]
- Valles, C.; Zhang, X.; Cao, J.; Lin, F.; Young, R.J.; Lombardo, A.; Ferrari, A.C.; Burk, L.; Mülhaupt, R.; Kinloch, I.A. Graphene/polyelectrolyte layer-by-layer coatings for electromagnetic interference shielding. ACS Nano 2019, 2, 5272–5281. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Zhang, M.; Ji, J.; Pang, X.; Liu, R. Three-dimensional heterostructured reduced graphene oxide-hexagonal boron nitride-stacking material for silicone thermal grease with enhanced thermally conductive properties. Nanomaterials 2019, 9, 938. [Google Scholar] [CrossRef] [Green Version]
- Yoonessi, M.; Gaier, J.R. Highly conductive multifunctional graphene polycarbonate nanocomposites. ACS Nano 2010, 4, 7211–7220. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Wu, Q.; Zhang, J.; Wu, M.; Chen, C.; Cao, Z. School of Chemistry and Chemical Engineering of Anhui University & Key Laboratory of Environment-friendly Polymer Materials of Anhui Province, Anhui University; Caishiji coating Ltd. Preparation and performance study of reduced silanized-grapheneoxide/waterborne polyurethane composites. Fine Chem. 2016, 33, 241–246. [Google Scholar]
- Chen, D.; Zhu, H.; Liu, T. In Situ thermal preparation of polyimide nanocomposite films containing functionalized graphene sheets. ACS Appl. Mater. Interface 2010, 2, 3702–3708. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, M.; Jana, T. Graphene nanosheets generated from sulfonated polystyrene/graphene nanocomposite. Compos. Sci. Technol. 2013, 87, 94–102. [Google Scholar] [CrossRef]



| Graphene Mass Fraction (wt%) | Oxygen Permeability (cm3/m2 24 h 0.1 MPa) |
|---|---|
| 0.0 | 213.4 ± 3.6 |
| 0.5 | 208.1 ± 2.1 |
| 1.0 | 205.2 ± 1.0 |
| 1.5 | 160.2 ± 5.5 |
| 2.0 | 203.2 ± 3.2 |
| 2.5 | 231.5 ± 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, X.; Chen, M.; Fu, J.; Lin, Z.; Li, Y.; Wu, J.; Yan, J.; Chen, X.; Ge, J. Eugenol Polysiloxane-Polycarbonate/Graphene Nanocomposite: Enhanced in Thermostability and Barrier Property. Nanomaterials 2019, 9, 1747. https://doi.org/10.3390/nano9121747
Pang X, Chen M, Fu J, Lin Z, Li Y, Wu J, Yan J, Chen X, Ge J. Eugenol Polysiloxane-Polycarbonate/Graphene Nanocomposite: Enhanced in Thermostability and Barrier Property. Nanomaterials. 2019; 9(12):1747. https://doi.org/10.3390/nano9121747
Chicago/Turabian StylePang, Xiaoyan, Mingde Chen, Junwei Fu, Zehua Lin, Yuming Li, Jianxin Wu, Jie Yan, Xunjun Chen, and Jianfang Ge. 2019. "Eugenol Polysiloxane-Polycarbonate/Graphene Nanocomposite: Enhanced in Thermostability and Barrier Property" Nanomaterials 9, no. 12: 1747. https://doi.org/10.3390/nano9121747





