Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops
Abstract
:1. Introduction
1.1. Economic Importance of Post-Harvest Diseases
1.2. Problems of Synthetic Fungicides
1.3. Alternative Control Means and Their Mode of Action
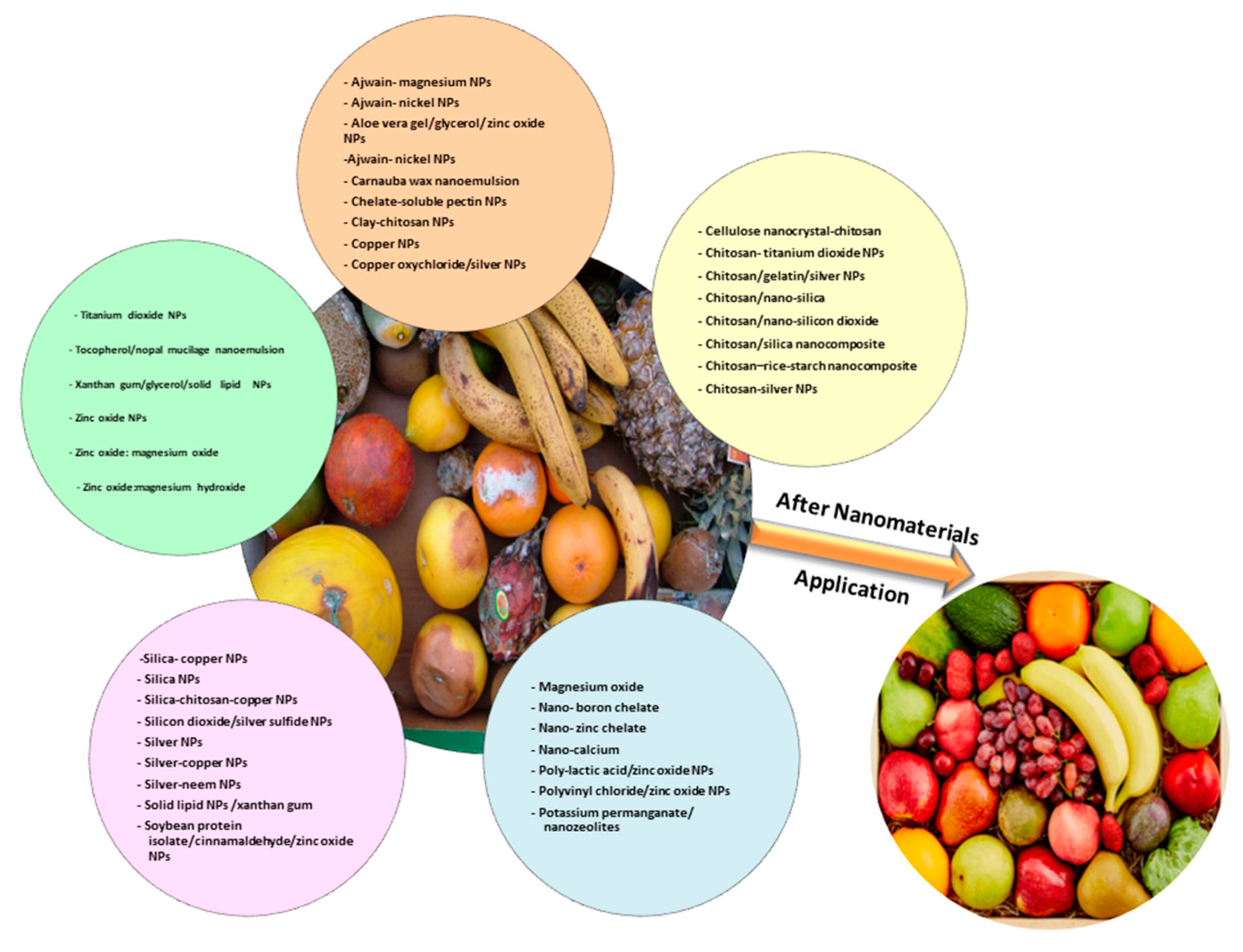
1.4. Nanomaterials as Candidate to Reduce Fungicides Use
2. Post-Harvest Diseases of Citrus
3. Post-Harvest Diseases of Grapes
4. Post-Harvest Diseases of Banana
5. Post-Harvest Diseases of Apple
6. Peach and Nectarine
7. Mango
8. Apricot, Guava, Avocado, Papaya and Dragon Fruits
9. Pear, Longan, Loquat, Jujube and Pomegranate Fruits
10. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feregrino-Perez, A.A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Teng, P.S.; Krupa, S.V. Assessment of losses which constrain production and crop improvement in agriculture and forestry. In Proceedings of the E. C. Stackman Commemorative Symposium, Minneapolis, MN, USA, 6–7 November 1980; p. 327. [Google Scholar]
- Teng, P.S. Crop Loss Assessment and Pest Management; Teng, P.S., Ed.; APS Press: Eagan, MN, USA, 1987; p. 270. [Google Scholar]
- Oerke, E.C.; Dehne, H.W.; Schönbeck, F.; Weber, A. Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Ladaniya, M.S. Citrus Fruit; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of post-harvest gray mold of ‘Italia’ table grapes. Post-Harvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R. Salt strategies to control Botrytis mold of’ Benitaka’ table grapes and to maintain fruit quality during storage. Post-Harvest Biol. Technol. 2014, 95, 95–102. [Google Scholar] [CrossRef]
- Gastavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Stammler, G.; Brix, H.D.; Nave, B.; Gold, R.; Schoefl, U. Studies on the biological performance of boscalid and its mode of action. In Proceedings of the Modern Fungicides and Antifungal Compounds V, Friedrichroda, Germany, 6–10 May 2007; Dehne, H.W., Deising, H.B., Gisi, U., Kuck, K.H., Russell, P.E., Lyr, H., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2008; pp. 45–51. [Google Scholar]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea (Gray Mold). In Post-Harvest Decay; Academic Press: New York, NY, USA, 2014; pp. 131–146. [Google Scholar]
- Palou, L. Post-harvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Fallanaj, F.; Sanzani, S.M.; Zavanella, C.; Ip polito, A. Salt addition improves the control of citrus post-harvest diseases using electrolysis with conductive diamond electrodes. J. Plant Pathol. 2013, 95, 373–383. [Google Scholar]
- Hao, W.; Li, H.; Hu, M.; Yang, L.; Rizwan-ul-Haq, M. Integrated control of citrus green and blue mold and sour rot by Bacillus amyloliquefaciens in combination with tea saponin. Post-Harvest Biol. Technol. 2011, 59, 316–323. [Google Scholar] [CrossRef]
- Sánchez-Torres, P.; Tuset, J.J. Molecular insights into fungicide resistance in sensitive and resistant Penicillium digitatum strains infecting citrus. Post-Harvest Biol. Technol. 2011, 59, 159–165. [Google Scholar] [CrossRef]
- Vitale, A.; Panebianco, A.; Polizzi, G. Baseline sensitivity and efficacy of fluopyram against Botrytis cinerea from table grape in Italy. Ann. Appl. Biol. 2016, 169, 36–45. [Google Scholar] [CrossRef]
- Piccirillo, G.; Carrieri, R.; Polizzi, G.; Azzaro, A.; Lahoz, E.; Fernández-Ortuño, D.; Vitale, A. in vitro and in vivo activity of QoI fungicides against Colletotrichum gloeosporioides causing fruit anthracnose in Citrus sinensis. Sci Hortic. 2018, 236, 90–95. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, K.; Ligorio, A.; Nigro, F.; Ippolito, A. Activity of salts incorporated in wax in controlling post-harvest diseases of citrus fruit. Post-Harvest Biol. Technol. 2012, 65, 39–43. [Google Scholar] [CrossRef]
- Youssef, K.; Ligorio, A.; Sanzani, S.M.; Nigro, F.; Ippolito, A. Control of storage diseases of citrus by pre- and post-harvest application of salts. Post-Harvest Biol. Technol. 2012, 72, 57–63. [Google Scholar]
- Youssef, K.; Sanzani, S.M.; Myrta, A.; Ippolito, A. Effect of a novel potassium bicarbonate-based formulation against Penicillium decay of oranges. J. Plant Pathol. 2014, 96, 419–424. [Google Scholar]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of post-harvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lachhab, N.; Sanzani, S.M.; Fallanaj, F.; Youssef, K.; Nigro, F.; Boselli, M.; Ippolito, A. Protein hydrolysates as resistance inducers for controlling green mould of citrus fruit. Acta Hortic. 2015, 1065, 1593–1598. [Google Scholar] [CrossRef]
- Fallanaj, F.; Sanzani, S.M.; Youssef, K.; Zavanella, C.; Salerno, M.G.; Ippolito, A. A new perspective in controlling post-harvest citrus rots: The use of electrolyzed water. Acta Hortic. 2015, 1065, 1599–1605. [Google Scholar] [CrossRef]
- Salem, E.A.; Youssef, K.; Sanzani, S.M. Evaluation of alternative means to control post-harvest Rhizopus rot of peaches. Sci. Hortic. 2016, 198, 86–90. [Google Scholar] [CrossRef]
- Jeong, R.D.; Chu, E.H.; Lee, G.W.; Cho, C.; Park, H.J. Inhibitory effect of gamma irradiation and its application for control of post-harvest green mold decay of Satsuma mandarins. Int. J. Food Microbiol. 2016, 234, 1–8. [Google Scholar] [CrossRef]
- Hussien, A.; Ahmed, Y.; Al-Essawy, A.H.; Youssef, K. Evaluation of different salt amended electrolysed water to control post-harvest moulds of citrus. Trop. Plant Pathol. 2018, 43, 10–20. [Google Scholar]
- Hussien, A.; Al-Essawy, A.; Abo Rehab, M.; Youssef, K. Preliminary investigation of alkaline and acidic electrolysed water to control Penicillium species of Citrus. Citrus Res. Technol. 2017, 38, 175–183. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; Colombo, R.C.; Canteri, M.G.; Abd-Elsalam, K.A. Acibenzolar-S-methyl against Botrytis mold on table grapes in vitro and in vivo. Agronomy Sci. Biotechnol. 2019, 5, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.; Hussien, A. Electrolysed water and salt solutions can reduce green and blue molds while maintain the quality properties of ‘Valencia’ late oranges. Post-Harvest Biol. Technol. 2020, 159, 111025. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for post-harvest disease control: The journey from simplicity to complexity. Post-Harvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Sanzani, S.M.; Ippolito, A. New techniques for managing post-harvest diseases of fruit. In Integrated Management of Diseases and Insect Pests of Tree Fruit; Xu, X.M., Fountain, M., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; p. 748. [Google Scholar]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of post-harvest biocontrol research: Is it time for a new paradigm? Post-Harvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Ippolito, A.; Terry, L.A. Sodium carbonate and bicarbonate treatments induce resistance to post-harvest green mould on citrus fruit. Post-Harvest Biol. Technol. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Youssef, K.; Sanzani, S.M.; Ligorio, A.; Fallanaj, F.; Nigro, F.; Ippolito, A. Biochemical and transcriptomic changes associated with induced resistance in citrus fruits treated with sodium salts. Acta Hortic. 2015, 1065, 1627–1632. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R.; de Oliveira, A.G. Ultra-structural alterations in Botrytis cinerea−the causal agent of gray mold−treated with salt solutions. Biomolecules 2019, 9, 582. [Google Scholar] [CrossRef] [Green Version]
- Kloepper, J.; Tuzun, S.; Kuć, J. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 347–349. [Google Scholar] [CrossRef]
- Walters, D.R.; Newton, A.C.; Lyon, G.D. Induced resistance: Helping plants to help themselves. Biologist 2005, 52, 28–33. [Google Scholar]
- Sharma, H.C.; Crouch, J.H.; Sharma, K.K.; Seetharama, N.; Hash, C.T. Applications of biotechnology for crop improvement: Prospects and constraints. Plant Sci. 2002, 163, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Yamaoka, N.; Takeuchi, Y. Elicitors: Their Significance and Primary Modes of Action in the Induction of plant defence reactions. Plant Cell Physiol. 1993, 34, 1163–1173. [Google Scholar]
- Alghuthaymi, M.A.; Ali, A.A.; Hashim, A.F.; Abd-Elsalam, K.A. A Rapid Method for the Detection of Ralstonia solanacearum by Isolation DNA from Infested Potato Tubers Based on Magnetic Nanotools. Philipp. Agric. Sci. 2016, 99, 113–118. [Google Scholar]
- Sharma, S.; Jaiswal, S.; Duffy, B.; Jaiswal, A.K. Nanostructured Materials for Food Applications: Spectroscopy, Microscopy and Physical Properties. Bioengineering 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller., R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 October 2019).
- Ippolito, A.; Nigro, F. Agrumi (pp. 181–195). In Postharvest Pathology of Plant Products; De Cicco, V., Bertolini, P., Salerno, M.G., Eds.; Piccin Nuova Libraria SpA: Padova, Italy, 2009; p. 260. [Google Scholar]
- Youssef, K.; Ahmed, Y.; Ligorio, A.; D’Onghia, A.M.; Nigro, F.; Ippolito, A. First report of Penicillium ulaiense as a post-harvest pathogen of orange fruit in Egypt. Plant Pathol. 2010, 59, 1174. [Google Scholar] [CrossRef]
- Youssef, K.; Hashim, A.F. Inhibitory Effect of Clay/Chitosan Nanocomposite against Penicillium digitatum on Citrus and its Possible Mode of Action. Jordan J. Biolog. Sci. 2020, 13. in press. [Google Scholar]
- Kumar, S.; Jog, J.P.; Natarajan, U. Preparation and characterization of poly(methyl methacrylate)–clay nanocomposites via melt intercalation: The effect of organoclay on the structure and thermal properties. J. Appl. Polym. Sci. 2003, 89, 1186–1194. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lim, H.J.; Park, S.M.; Lee, S.J. Synthesis and characterization of high impact polystyrene/organically modified layered silicate nanocomposites. Polymer Korea 2003, 27, 377–384. [Google Scholar]
- Xu, Y.; Ren, X.; Hanna, M.A. Chitosan/Clay Nanocomposite Film Preparation and Characterization. J. Appl. Polym. Sci. 2006, 99, 1684–1691. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chatchawan, S. Bio stimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 195, 49–65. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Ebadollahi, A. Potential application of chitosan-clay coating on some quality properties of lemon during storage. Agric. Eng. Int. 2017, 19, 189–194. [Google Scholar]
- Khoshtaghaza, M.H.; Taghinezhad, E. Investigation effect of particle Nano coating on storage quality properties of Thomson orange. J. Food Sci. Technol. Mys. 2017, 13, 113–125. [Google Scholar]
- Xu, D.; Qin, H.; Ren, D. Prolonged preservation of tangerine fruits using chitosan/montmorillonite composite coating. Post-Harvest Biol. Technol. 2018, 143, 50–57. [Google Scholar] [CrossRef]
- Abdelmalek, G.A.M.; Salaheldin, T.A. Silver Nanoparticles as a Potent Fungicide for Citrus Phytopathogenic Fungi. J. Nanomed. Res. 2016, 3, 00065. [Google Scholar]
- Youssef, K.; Hashim, A.F.; Margarita, R.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Antifungal Efficacy of Chemically-Produced Copper Nanoparticles Against Penicillium digitatum and Fusarium solani on Citrus Fruit. Philip. Agric. Sci. 2017, 100, 69–78. [Google Scholar]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copperbased nanoparticles: A review. Appl. Microbiol. Biotechnol. 2013, 98, 1001–1009. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Physiological effects and mode of action of ZnO nanoparticles against post-harvest fungal contaminants. Food Res. Int. 2017, 101, 274–279. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining post-harvest quality. Eur. J. Plant Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.H.; Kim, H.J.; Choi, S.H. A new composition of nanosized silica–silver for control of various plant diseases. Plant Pathol. J. 2006, 223, 295–302. [Google Scholar]
- Piña-Barrera, A.M.; Álvarez-Román, R.; Báez-González, J.G.; Amaya-Guerra, C.A.; Rivas-Morales, C.; Gallardo-Rivera, C.T.; Galindo-Rodríguez, S.A. Application of a multisystem coating based on polymeric nanocapsules containing essential oil of Thymus vulgaris L. to increase the shelf life of table grapes (Vitis vinifera L.). IEEE Trans. Nanobiosci. 2019. [Google Scholar] [CrossRef]
- Plascencia-Jatomea, M.; Yépiz-Gómez, M.S.; Velez-Haro, J.M. Aspergillus spp. (Black Mold). In Post-Harvest Decay, Control Strategies; Academic Press: New York, NY, USA, 2014; pp. 267–286. [Google Scholar]
- Fateixa, S.; Neves, M.C.; Almeida, A.; Oliveira, J.; Trindade, T. Anti-fungal activity of SiO2/Ag2S nanocomposites against Aspergillus niger. Colloids Surf. B. Biointerfaces. 2009, 74, 304–308. [Google Scholar] [CrossRef]
- Semeykina, A.L.; Skulachev, V.P. Submicromolar Ag+ increases passive Na+ permeability and inhibits the respiration-supported formation of Na+ gradient in Bacillus FTU vesicles. FEBS Lett. 1990, 269, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Keleher, J.; Bashant, J.; Heldt, N.; Johnson, L.; Li, Y. Photo-catalytic preparation of silver-coated TiO2 particles for antibacterial applications. World J. Microbiol. Biotechnol. 2002, 18, 133–139. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef]
- Ouda, S.M. Antifungal Activity of Silver and Copper Nanoparticles on Two Plant Pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Derbalah, A.S.; Elkot, G.A.; Hamza, A.M. Laboratory evaluation of botanical extracts, microbial culture filtrates and silver nanoparticles against Botrytis cinerea. Ann. Microbiol. 2012, 62, 1331–1337. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf. Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Melo, C.B.N.F.; de MendonçaSoares, B.L.; Diniz, K.M.; Leal, C.F.; Canto, D.; Flores, M.A.P.; da Costa Tavares-Filho, H.J.; Galembeck, A.; Stamford, M.T.L.; Stamford-Arnaud, T.M.; et al. Effects of fungal chitosan nanoparticles as eco-friendly edible coatings on the quality of post-harvest table grapes. Post-Harvest Biol. Technol. 2018, 139, 56–66. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Joyce, D.C.; Jiang, Y.; Qu, H.; Duan, X. Physiology and quality response of harvested banana fruit to cold shock. Post-Harvest Biol. Technol. 2010, 55, 154–159. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N.; Siddiqui, Y. Effect of a Novel Edible Composite Coating Based on Gum Arabic and Chitosan on Biochemical and Physiological Responses of Banana Fruits during Cold Storage. J. Agric. Food Chem. 2011, 59, 5474–5482. [Google Scholar] [CrossRef]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 368, 8–63. [Google Scholar] [CrossRef]
- Esyanti, R.R.; Zaskia, H.; Amalia, A.; Nugrahapraja, d.H. Chitosan Nanoparticle-based coating as post-harvest technology in banana. J. Phys. Conf. Ser. 2019, 1204, 012109. [Google Scholar] [CrossRef]
- Jagana, D.; Hegde, Y.R.; Lella, R. Green nanoparticles: A novel approach for the management of banana anthracnose caused by Colletotrichum musae. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1749–1756. [Google Scholar] [CrossRef]
- Lustriane, C.; Dwivany, F.M.; Suendo, V.; Reza, M. Effect of chitosan and chitosan-nanoparticles on postharvest quality of banana fruits. J. Plant Biotechnol. 2018, 45, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, Q.; Sun, Y.; Chen, B.; Wu, X.; Le, T. Improvement of banana post-harvest quality using a novel soybean protein isolate/cinnamaldehyde/zinc oxide bionanocomposite coating strategy. Sci. Hortic. 2019, 258, 108786. [Google Scholar] [CrossRef]
- Martelli, M.R.; Barros, T.T.; de Moura, M.R.; Mattoso, L.H.; Assis, O.B. Effect of chitosan nanoparticles and pectin content on mechanical properties and water vapor permeability of banana puree films. J. Food Sci. 2013, 78, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and post-harvest shelf-life extension of apple cv. Golab Kohanz. LWT-Food Sci. Technol. 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosan nanoparticle coatings reduce microbial growth on fresh-cut apples while not affecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Jiang, Y.; Ding, Y.; Yun, J.; Yao, T.; Zhang, P. Effect of nano-ZnO-coated active packaging on quality of fresh-cut ‘Fuji’ apple. Int. J. Food Sci. Technol. 2011, 46, 1947–1955. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA Film Incorporated with ZnO Nanoparticle on the Quality Attributes of Fresh-Cut Apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on post-harvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Sardella, D.; Gatt, R.; Valdramidis, V.P. Assessing the efficacy of zinc oxide nanoparticles against Penicillium expansum by automated turbidimetric analysis. Mycology 2018, 9, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Del Real, A.; González-Reza, R.M.; Galindo-Pérez, M.J.; Quintanar-Guerrero, D. Fresh-cut red delicious apples coating using tocopherol/mucilage nanoemulsion: Effect of coating on polyphenol oxidase and pectin methylesterase activities. Food Res. Int. 2014, 62, 974–983. [Google Scholar] [CrossRef]
- Kaur, M.; Kalia, A.; Thakur, A. Effect of Biodegradable Chitosan-rice-starch Nanocomposite Films on Post-Harvest Quality of Stored Peach Fruit. Starch 2017, 69, 1600208. [Google Scholar] [CrossRef] [Green Version]
- Masoumeh, E.; Behzad, G.; Yousef, R.K.; Mehrnaz, E.; Naser, B. Effect of the Potassium Permanganate Coated Zeolite Nanoparticles on the Quality Characteristic and Shelf Life of Peach and Nectarine. J. Agric. Technol. 2015, 1, 1263–1273. [Google Scholar]
- Gad, M.M.; Zagzog, O.A.; Hemeda, O.M. Development of Nano-Chitosan Edible Coating for Peach Fruits Cv. Desert Red. Int. J. Environ. 2016, 5, 43–55. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdappa, P.; Gowda, S.; Chethana, C.S.; Madhura, S. Antifungal activity of chitosan-silver nanoparticle composite against Colletotrichum gloeosporoides associated with mango anthracnose. Afr. J. Microbiol. Res. 2014, 8, 1803–1812. [Google Scholar]
- Dubey, P.K.; Shukla, R.N.; Srivastava, G.; Mishra, A.A.; Pandey, A. Study on Quality Parameters and Storage Stability of Mango Coated with Developed Nanocomposite Edible Film. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2899–2935. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Raghu, H.S.; Divyashree, K.; Rajeshwara, A.N. Antifungal efficiency of copper oxychloride-conjugated silver nanoparticles against Colletotrichum gloeosporioides which causes anthracnose disease. Asian J. Pharm. Clin. Res. 2019, 12, 230–233. [Google Scholar]
- Liu, H.; Chen, F.; Yang, H.; Yao, Y.; Gong, X.; Xin, Y.; Ding, C. Effect of calcium treatment on nanostructure of chelate-soluble pectin and physicochemical and textural properties of apricot fruits. Food Res. Int. 2009, 42, 1131–1140. [Google Scholar] [CrossRef]
- Emadpour, M.; Kalaj, Y.R. Effect of ethylene absorption using nano-particles on the storage and quality characteristics of apricot. Agron. Hortic. 2009, 21, 82–89. [Google Scholar]
- Garcia-Betanzos, C.I.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Galindo-Pérez, M.J.; Zambrano-Zaragoza, M.L. Influence of solid lipid nanoparticle/xanthan gum coatings on compositional and enzymatic changes in guava (Psidium guajava L.) during ripening. Acta. Hortic. 2018, 1194, 289–296. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Ramirez-Zamorano, P.; Cornejo-Villegas, M.; Gutiérrez-Cortez, E.; Quintanar-Guerrero, D. Use of solid lipid nanoparticles (SLNs) in edible coatings to increase guava (Psidium guajava L.) shelf-life. Food Res. Int. 2013, 51, 946–953. [Google Scholar] [CrossRef]
- González-Reza, R.M.; Pérez-Olivier, M.S.; Miranda-Linares, V.; Zambrano-Zaragoza, M.L. Effect of solid lipid nanoparticles coating on shelf life of refrigerated fresh-cut guava. Acta. Hortic. 2018, 1194, 553–560. [Google Scholar] [CrossRef]
- Martinez-Ortiz, M.A.; Palma-Rodriguez, H.M.; Montalvo-Gonzalez, E.; Sayago-Ayerdi, S.G.; Utrilla-Coello, R.; Vargas-Torres, A. Effect of using microencapsulated ascorbic acid in coatings based on resistant starch chayotextle on the quality of guava fruit. Sci. Hortic. 2019, 256, 108604. [Google Scholar] [CrossRef]
- De la Rosa-García, S.C.; Martínez-Torres, P.; Gómez-Cornelio, S.; Corral-Aguado, M.A.; Quintana, P.; Gómez-Ortíz, N.M. Antifungal Activity of ZnO and MgO Nanomaterials and Their Mixtures against Colletotrichum gloeosporioides Strains from Tropical Fruit. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Adnan, A.; Mohammad, R.; Farhan, J.A.; Zeenat, I.; Roop, K.K.; Aqil, M.; Sushama, T. Nanoemulsion components screening and selection: A technical note. AAPS Pharm. Sci. Tech. 2009, 10, 69–76. [Google Scholar]
- Zahid, N.; Ali, A.; Manickam, S.; Siddiqui, Y.; Maqboo, M. Potential of chitosan-loaded nanoemulsions to control different Colletotrichum spp. and maintain quality of tropical fruits during cold storage. J. Appl. Microbiol. 2012, 113, 925–939. [Google Scholar]
- Ohashi, T.L.; Pilon, L.; Spricigo, P.C.; Miranda, M.; Correa, D.S.; Ferreira, M.D. Post-harvest quality of ‘Golden’ papayas (Carica papaya L.) coated with carnauba wax nanoemulsions. Rev. Iber. Tecnología Postcosecha. 2015, 16, 199–209. [Google Scholar]
- Zahid, N.; Alderson1, P.G.; Ali, A.; Maqbool, M.; Manickam, S. in vitro Control of Colletotrichum gloeosporioides by Using Chitosan Loaded Nanoemulsions. Acta Hortic. 2013, 1012, 769–774. [Google Scholar]
- Deng, Z.; Jung, J.; Simonsen, J.; Wang, Y.; Zhao, Y. Cellulose Nanocrystal Reinforced Chitosan Coatings for Improving the Storability of Post-harvest Pears Under Both Ambient and Cold Storages. J. Food Sci. 2017, 82, 453–462. [Google Scholar] [CrossRef]
- Shi, S.; Wanga, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yanga, L.; Zhang, Y. Effects of chitosan/nano-silica on post-harvest quality and antioxidant capacity of loquat fruit during cold storage. Post-Harvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Davarpanaha, S.; Tehranifara, A.; Davarynejada, G.; Abadíab, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers on pomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hortic. 2016, 210, 57–64. [Google Scholar] [CrossRef] [Green Version]
| Fruit/Cultivar | Scientific Name | Nanomaterial | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Valencia Late orange | Citrus sinensis L. Osb. | Clay-chitosan nanocomposite | Antifungal | NM * | [49] |
| Lemon | Citrus limon L. Osb. | Chitosan-clay nanocomposite | Coating | NM * | [54] |
| Thomson navel orange | C. sinensis L. Osb. | Chitosan-clay nanocomposite | Edible coating | NM * | [55] |
| Tangerine | Citrus tangerine Hort. ex Tanaka | Chitosan/montmorillonite | Coating/antifungal | NM * | [56] |
| Washington navel orange | C. sinensis L. Osb. | Silver nanoparticles | Antifungal | 10 ± 5 | [57] |
| Valencia Late | C. sinensis L. Osb. | Copper nanoparticles | Antifungal | 48 | [58] |
| - | C. sinensis L. Osb. | ZnO nanoparticles | Antifungal | <50 | [60] |
| Lemon | C. limon L. Osb. | TiO2 nanoparticles | Coating/antifungal | 7 | [61] |
| Cultivar/Type | Scientific Name | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Flame Seedless table grape | Vitis vinifera L. | Silica nanoparticles (NPs), Copper NPs, Silica-copper NPs, Chitosan NPs | Antifungal | Si (140–150) Cu (25–35) Si-Cu (520–550) | [63] |
| Italia and Benitaka table grapes | V. vinifera L. | Chitosan/silica nanocomposite | Antifungal | 48 | [64] |
| Grapes | V. vinifera L. | Nanocapsules/Thymus vulgaris L. | Coating/antifungal | 153.9 | [66] |
| - | V. vinifera L. | SiO2/Ag2S nanocomposites | Antifungal | 300 | [68] |
| - | V. vinifera L. | ZnO-nanoparticles | Antifungal | 70 ± 15 | [71] |
| - | V. vinifera L. | Silver-nanoparticles, copper-nanoparticles and silver-copper-nanoparticles | Antifungal | Silver (38) Copper (20) | [72] |
| - | V. vinifera L. | Silver-nanoparticles | Antifungal | 50 | [73] |
| Red grapes | V. vinifera L. | Chitosan-TiO2 composite | Antimicrobial | 50–80 | [74] |
| Red grapes | V. vinifera L. | Chitosan/gelatin and silver nanoparticles | Coating | 25–45 | [75] |
| Grapes | Vitis labrusca L. | Chitosan nanoparticles | Edible coating | 128.3 | [76] |
| Group/Cultivar | Scientific Name | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| - | Musa acuminata L. | Chitosan/gum arabic | Coating | NM * | [78] |
| Cavendish bananas AAA group | M. acuminata L. | Chitosan-nanoparticles | Edible coating | 102.4–370 | [80] |
| - | M. acuminata L. | Ajwain-magnesium nanoparticles, Ajwain-nickel nanoparticles and Silver-neem nanoparticles | Antifungal | 68 | [81] |
| M. acuminata AAA group | M. acuminata L. | Chitosan-nanoparticles | Edible coating | 121.2 | [82] |
| - | M. acuminata L. | Soybean protein isolate/cinnamaldehyde/ZnO nanoparticles | Antifungal/film coating | NM * | [83] |
| Nanica | Musa cavendishii Lamb. | Chitosan-nanoparticles | Coating film | 88.79 | [84] |
| Cultivar | Scientific Name | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Golab Kohanz | Malus domestica Bork. | Chitosan-nanoemulsion | Coating | ≤100 | [85] |
| Gala | M. domestica Bork. | Chitosan-nanoparticles | Coatings/antimicrobial | 110–300 | [86] |
| Fuji | M domestica Bork. | Polyvinyl chloride/ZnO nanoparticle | Nanopackaging coating | 200–400 | [87] |
| Yunnan ZhaoTong | M. domestica Bork. | Poly-lactic acid/ZnO nanoparticle | Nanopackaging coating | NM * | [88] |
| Red Delicious | M. domestica Bork. | Nano-calcium | Coating | NM * | [89] |
| - | M. domestica Bork. | ZnO nanoparticles | Antifungal | 70 ± 15 | [71] |
| - | M. domestica Bork. | ZnO nanoparticles | Antifungal | <50 | [90] |
| Red Delicious | M. domestica Bork. | Tocopherol/nopal mucilage nanoemulsion | Encapsulant | <1000 | [91] |
| Cultivar | Scientific Name | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Shan-i-Punjab peach | Prunus persica L. | Chitosan–rice-starch nanocomposite | Antimicrobial | NM * | [92] |
| Red Top and Anjiry peaches | P. persica L. | Potassium permanganate coated with nanozeolites | Removal of the ethylene | NM * | [93] |
| Red Gold, Songlu and Independence nectarines | P. persica var. nucipersica L. | Potassium permanganate coated with nanozeolites | Removal of the ethylene | NM * | [93] |
| Desert Red peach | P. persica L. | Chitosan nanoparticles | Antifungal | 50 | [94] |
| Cultivar | Scientific Name | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Alphonso | Magnifera indica L. | Chitosan-silver nanoparticle composite | Antifungal | 495–616 | [96] |
| Dasheri | M. indica L. | Aloe vera gel, glycerol and ZnO nanoparticles | Film coating | NM * | [97] |
| - | M. indica L. | Copper oxychloride-conjugated AgNPs | Antifungal | 21–25 | [98] |
| Fruit | Scientific Name | Cultivar | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|---|
| Apricot | Prunus armeniaca L. | Jinhong | Chelate-soluble pectin nanostructural | Coating | NM * | [99] |
| Apricot | P armeniaca L. | - | Potassium permanganate coated with nanozeolites | Extend shelf life | NM * | [100] |
| Guava | Psidium guajava L. | Media China | Solid lipid nanoparticles/xanthan gum | Coating | 276 | [101] |
| Guava | P. guajava L. | Media China | Xanthan gum, glycerol and solid lipid nanoparticles | Coating | 245 | [102] |
| Guava | P. guajava L. | - | Microencapsulated starch/ascorbic acid | Coating | NM * | [104] |
| Avocado | Persea americana | in vitro | ZnO, MgO and ZnO: MgO and ZnO:Mg(OH)2 | Antifungal | 52–219 | [105] |
| Carica papaya | ||||||
| Banana | Musa acuminata L. | Pisang Berangan | Chitosan-loaded nanoemulsions | Antifungal | 200–1000 | [107] |
| Papaya | C. papaya L. | AAA Group Solo | ||||
| Dragon | Hylocereus polyrhizus | Red | ||||
| Jaina | ||||||
| Papaya | C. papaya L. | Golden | Carnauba wax nanoemulsions | Coating | 42 | [108] |
| Dragon | H. polyrhizus | in vitro | Chitosan-loaded nanoemulsions | Antifungal | 200–1000 | [109] |
| Fruit | Scientific Name | Cultivar | Nanomaterials | Objective | Size (nm) | Ref. |
|---|---|---|---|---|---|---|
| Pear | Pyrus communis L. | D’Anjou and Bartlett | Cellulose nanocrystal-chitosan | Coating | NM * | [110] |
| Longan | Dimocarpus longan Lour. | Shijia | Chitosan/nano-silica | Coating | NM * | [111] |
| Loquat | Eriobotrya japonica Lindl. | Baiyu | Chitosan/nano-silica | Coating | NM * | [112] |
| Jujubes | Ziziphus jujuba Mill. | Dongzao | Chitosan film/nano-silicon dioxide | Coating | NM * | [113] |
| Pomegranate | Punica granatum | Ardestani | Nano-zinc chelate and nano-boron chelate | Nutrients | 23–80 | [114] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffo Roberto, S.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops. Nanomaterials 2019, 9, 1752. https://doi.org/10.3390/nano9121752
Ruffo Roberto S, Youssef K, Hashim AF, Ippolito A. Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops. Nanomaterials. 2019; 9(12):1752. https://doi.org/10.3390/nano9121752
Chicago/Turabian StyleRuffo Roberto, Sergio, Khamis Youssef, Ayat Farghily Hashim, and Antonio Ippolito. 2019. "Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops" Nanomaterials 9, no. 12: 1752. https://doi.org/10.3390/nano9121752
APA StyleRuffo Roberto, S., Youssef, K., Hashim, A. F., & Ippolito, A. (2019). Nanomaterials as Alternative Control Means Against Postharvest Diseases in Fruit Crops. Nanomaterials, 9(12), 1752. https://doi.org/10.3390/nano9121752








