Rapid Water Softening with TEMPO-Oxidized/Phosphorylated Nanopapers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNF
2.2.1. Preparation of Unmodified CNF
2.2.2. Preparation of Phosphorylated TEMPO-CNF
2.3. Characterization of CNF
2.4. Preparation of Nanopapers
2.5. Characterization of Nanopapers
2.5.1. Determination of Zeta-Potential of Nanopapers
2.5.2. Morphology of the Cellulose Nanopapers
2.5.3. Specific Surface Area of Nanopapers by Nitrogen Sorption Experiments
2.5.4. Tensile Properties of Nanopapers
2.5.5. Thermal Degradation Behavior of the Papers
2.5.6. Pure Water Permeance and Ion Adsorption Performance of Nanopapers
3. Results and Discussion
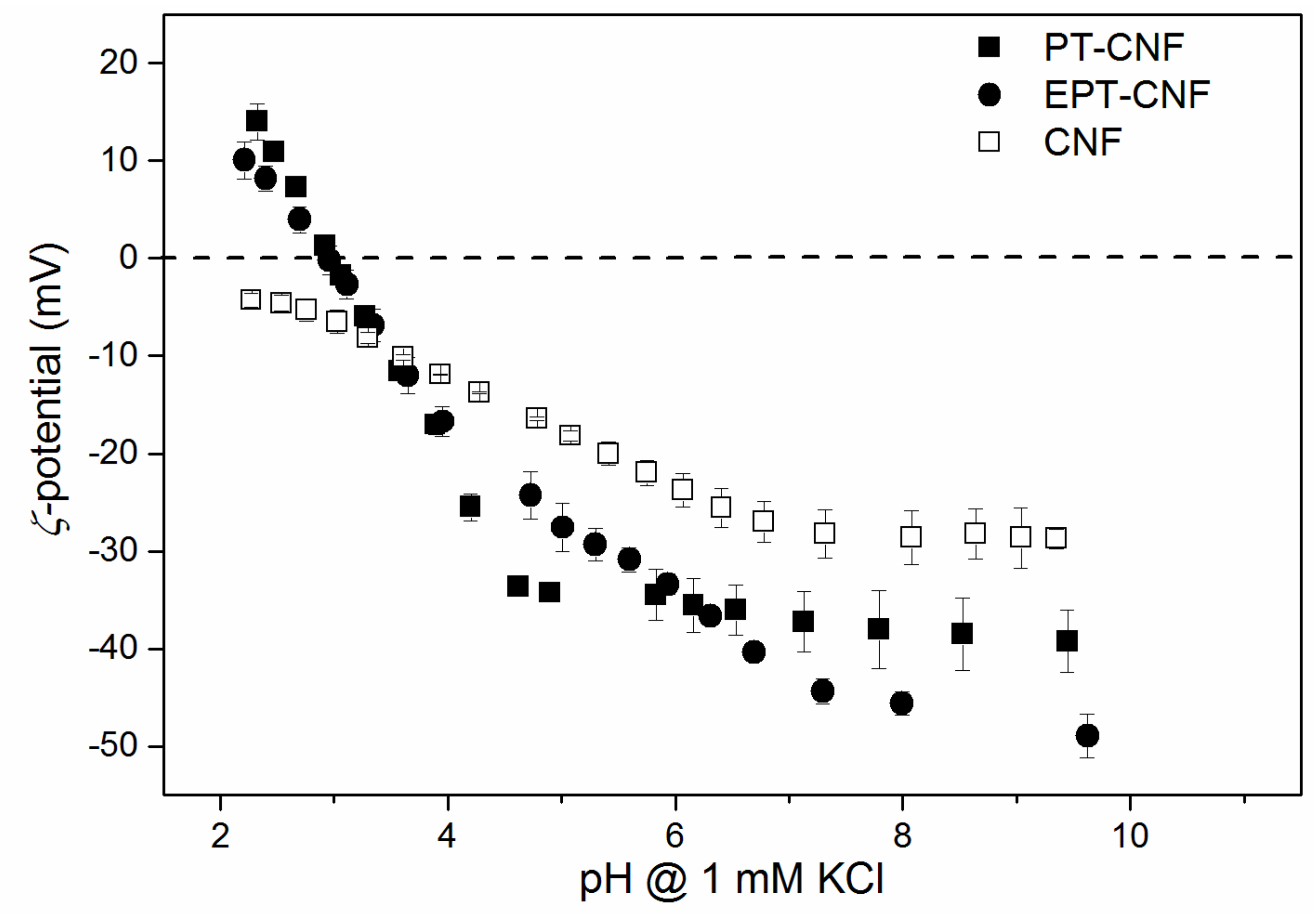
3.1. Preparation and Characterization of CNF and Nanopapers
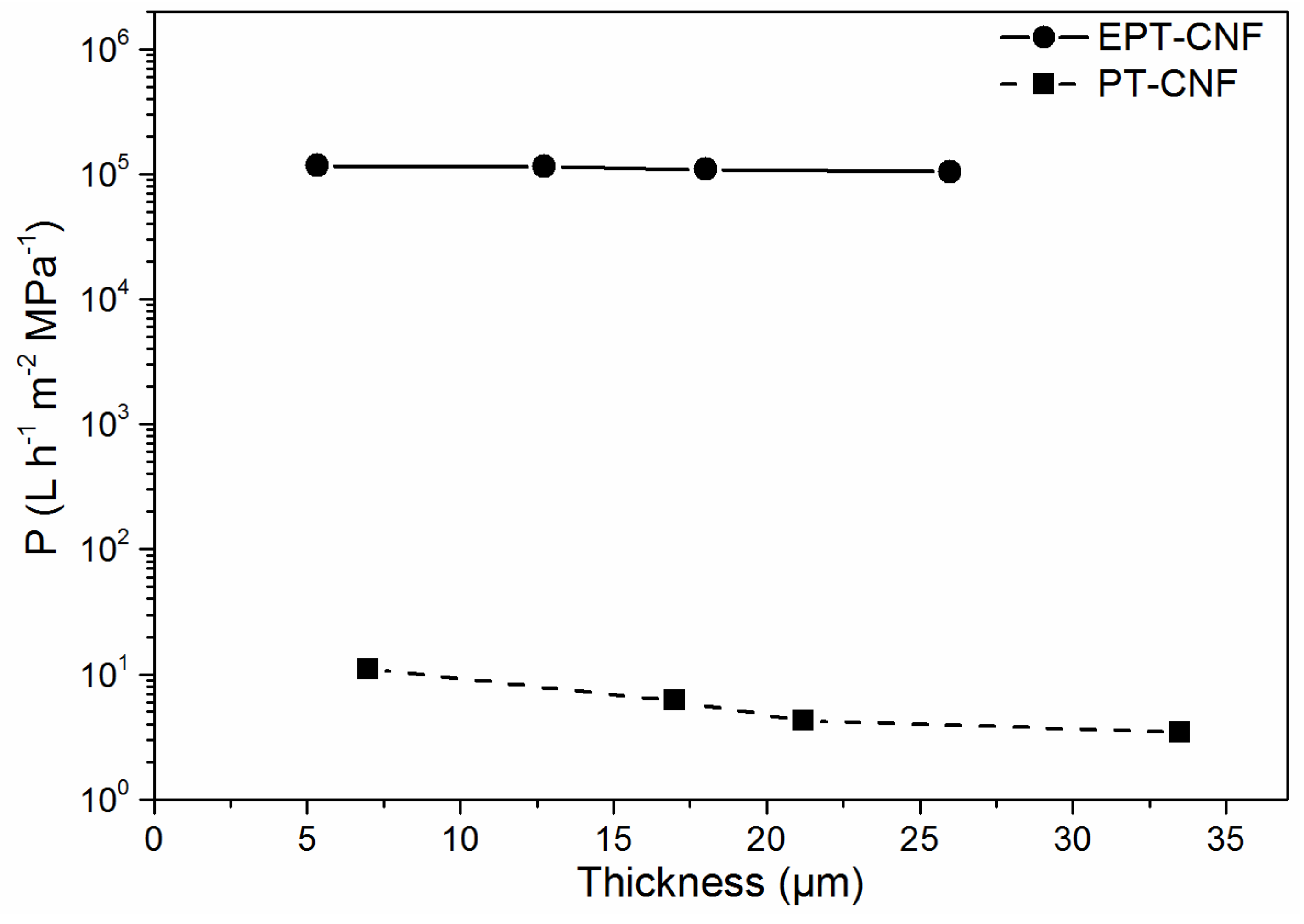
3.2. Permeance and Water Softening Performance of CNF Nanopapers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brastad, K.S.; He, Z. Water softening using microbial desalination cell technology. Desalination 2013, 309, 32–37. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Permanent hard water softening using carbon nanotube sheets. Desalination 2011, 268, 208–213. [Google Scholar] [CrossRef]
- Park, J.-S.; Song, J.-H.; Yeon, K.-H.; Moon, S.-H. Removal of hardness ions from tap water using electromembrane processes. Desalination 2007, 202, 1–8. [Google Scholar] [CrossRef]
- Gabrielli, C.; Maurin, G.; Francy-Chausson, H.; Thery, P.; Tran, T.T.M.; Tlili, M. Electrochemical water softening: Principle and application. Desalination 2006, 201, 150–163. [Google Scholar] [CrossRef]
- McNally, N.J.; Williams, H.C.; Phillips, D.R.; Smallman-Raynor, M.; Lewis, S.; Venn, A.; Britton, J. Atopic eczema and domestic water hardness. Lancet 1998, 352, 527–531. [Google Scholar] [CrossRef]
- Perkin, M.R.; Craven, J.; Logan, K.; Strachan, D.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.H.I.; Lack, G.; et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J. Allergy Clin. Immunol. 2016, 138, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Li, Z.; Ma, J.; Bai, X.; Kang, Y.; Hao, W.; Li, R. Effective removal of Mg2+ and Ca2+ ions by mesoporous LTA zeolite. Desalination 2014, 341, 10–18. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Kim, S.; Yoon, J. Electrochemical softening using capacitive deionization (CDI) with zeolite modified carbon electrode (ZMCE). Desalin. Water Treat. 2016, 57, 24682–24687. [Google Scholar] [CrossRef]
- Apell, J.N.; Boyer, T.H. Combined ion exchange treatment for removal of dissolved organic matter and hardness. Water Res. 2010, 44, 2419–2430. [Google Scholar] [CrossRef]
- Behrman, A.S. Recent Developments in Zeolite Softening. Ind. Eng. Chem. 1927, 19, 445–447. [Google Scholar] [CrossRef]
- Gans, R. Verfahren zur Behandlung von Wasser für häusliche und gewerbliche Zwecke. German Patent 197,111, October 1906. [Google Scholar]
- Gans, R. Verfahren zur Herstellung von wasserhaltigen Erdalkalialuminatsilikaten oder künstlichen Zeolithen. German Patent 174,097, January 1905. [Google Scholar]
- Entezari, M.H.; Tahmasbi, M. Water softening by combination of ultrasound and ion exchange. Ultrason. Sonochem. 2009, 16, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, I.; Catalfamo, P.; Cavallari, L.; Di Pasquale, S. Use of zeolitized pumice waste as a water softening agent. J. Hazard. Mater. 2007, 147, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, M.N.; Zarrabi, M.; Kazemian, H.; Amrane, A.; Yaghmaian, K.; Ghaffari, H.R. Removal of hardness agents, calcium and magnesium, by natural and alkaline modified pumice stones in single and binary systems. Appl. Surf. Sci. 2013, 274, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Bekri-Abbes, I.; Bayoudh, S.; Baklouti, M. The removal of hardness of water using sulfonated waste plastic. Desalination 2008, 222, 81–86. [Google Scholar] [CrossRef]
- Saleh, M.M. Water softening using packed bed of polypyrrole from flowing solutions. Desalination 2009, 235, 319–329. [Google Scholar] [CrossRef]
- Seo, S.-J.; Jeon, H.; Lee, J.K.; Kim, G.-Y.; Park, D.; Nojima, H.; Lee, J.; Moon, S.-H. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res. 2010, 44, 2267–2275. [Google Scholar] [CrossRef]
- Tuan, T.N.; Chung, S.; Lee, J.K.; Lee, J. Improvement of water softening efficiency in capacitive deionization by ultra purification process of reduced graphene oxide. Curr. Appl. Phys. 2015, 15, 1397–1401. [Google Scholar] [CrossRef]
- Fu, L.; Wang, J.; Su, Y. Removal of low concentrations of hardness ions from aqueous solutions using electrodeionization process. Sep. Purif. Technol. 2009, 68, 390–396. [Google Scholar] [CrossRef]
- Kabay, N.; Demircioglu, M.; Ersöz, E.; Kurucaovali, I. Removal of calcium and magnesium hardness by electrodialysis. Desalination 2002, 149, 343–349. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; der Bruggen, B.V. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]
- Lee, H.-J.; Song, J.-H.; Moon, S.-H. Comparison of electrodialysis reversal (EDR) and electrodeionization reversal (EDIR) for water softening. Desalination 2013, 314, 43–49. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, H.; Yang, Z.; Fu, C.-E.; Tian, Z.; Yang, W. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Liu, G.; Xue, M.; Yang, H. Polyether copolymer as an environmentally friendly scale and corrosion inhibitor in seawater. Desalination 2017, 419, 133–140. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Xu, B.; Yin, X.; Chen, Y.; Liu, Y.; Ji, Y.; Huan, Y. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 2017, 416, 166–174. [Google Scholar] [CrossRef]
- Bodzek, M.; Koter, S.; Wesołowska, K. Application of membrane techniques in a water softening process. Desalination 2002, 145, 321–327. [Google Scholar] [CrossRef]
- Bequet, S.; Abenoza, T.; Aptel, P.; Espenan, J.M.; Remigy, J.C.; Ricard, A. New composite membrane for water softening. Desalination 2000, 131, 299–305. [Google Scholar] [CrossRef]
- Anim-Mensah, A.R.; Krantz, W.B.; Govind, R. Studies on polymeric nanofiltration-based water softening and the effect of anion properties on the softening process. Eur. Polym. J. 2008, 44, 2244–2252. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Tung, K.-L.; Lee, D.-J.; Wang, D.-M.; Nanda, D.; Tung, K.-L.; Hsiung, C.-C.; Chuang, C.-J.; Ruaan, R.-C.; Chiang, Y.-C.; et al. Effect of solution chemistry on water softening using charged nanofiltration membranes. Desalination 2008, 234, 344–353. [Google Scholar]
- Rajabzadeh, S.; Liu, C.; Shi, L.; Wang, R. Preparation of low-pressure water softening hollow fiber membranes by polyelectrolyte deposition with two bilayers. Desalination 2014, 344, 64–70. [Google Scholar] [CrossRef]
- Zhao, F.-Y.; An, Q.-F.; Ji, Y.-L.; Gao, C.-J. A novel type of polyelectrolyte complex/MWCNT hybrid nanofiltration membranes for water softening. J. Membr. Sci. 2015, 492, 412–421. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Xu, Z.-L.; Ding, H.; Tang, Y.-J. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+. Desalination 2017, 420, 158–166. [Google Scholar] [CrossRef]
- Wang, D.; Yu, H.; Fan, X.; Gu, J.; Ye, S.; Yao, J.; Ni, Q. High Aspect Ratio Carboxylated Cellulose Nanofibers Cross-linked to Robust Aerogels for Superabsorption–Flocculants: Paving Way from Nanoscale to Macroscale. ACS Appl. Mater. Interfaces 2018, 10, 20755–20766. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, M.A.; Lizundia, E.; Moser, S.; Niederberger, M.; Nyström, G. Titania-Cellulose Hybrid Monolith for In-Flow Purification of Water under Solar Illumination. ACS Appl. Mater. Interfaces 2018, 10, 29599–29607. [Google Scholar] [CrossRef] [PubMed]
- Mautner, A.; Maples, H.A.; Sehaqui, H.; Zimmermann, T.; Perez de Larraya, U.; Mathew, A.P.; Lai, C.Y.; Li, K.; Bismarck, A. Nitrate removal from water using a nanopaper ion-exchanger. Environ. Sci. Water Res. 2016, 2, 117–124. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Mautner, A.; Mayer, F.; Hervy, M.; Lee, K.-Y.; Bismarck, A. Better together: Synergy in nanocellulose blends. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170043. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, P.A. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.Y.; Lahtinen, P.; Hakalahti, M.; Tammelin, T.; Li, K.; Bismarck, A. Nanopapers for organic solvent nanofiltration. Chem. Commun. 2014, 50, 5778–5781. [Google Scholar] [CrossRef] [Green Version]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mautner, A.; Lee, K.-Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.J.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Hakalahti, M.; Mautner, A.; Johansson, L.-S.; Hänninen, T.; Setälä, H.; Kontturi, E.; Bismarck, A.; Tammelin, T. Direct Interfacial Modification of Nanocellulose Films for Thermoresponsive Membrane Templates. ACS Appl. Mater. Interfaces 2016, 8, 2923–2927. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Claudpierre, S.; Grahn, M.; Oksman, K.; Mathew, A.P. Nanocellulose based functional membranes for water cleaning: Tailoring of mechanical properties, porosity and metal ion capture. J. Membr. Sci. 2016, 514, 418–428. [Google Scholar] [CrossRef]
- Mautner, A.; Maples, H.A.; Kobkeatthawin, T.; Kokol, V.; Karim, Z.; Li, K.; Bismarck, A. Phosphorylated nanocellulose papers for copper adsorption from aqueous solutions. Int. J. Environ. Sci. Technol. 2016, 13, 1861–1872. [Google Scholar] [CrossRef] [Green Version]
- Mautner, A.; Kobkeatthawin, T.; Bismarck, A. Efficient continuous removal of nitrates from water with cationic cellulose nanopaper membranes. Res. Eff. Technol. 2017, 3, 22–28. [Google Scholar] [CrossRef]
- Ferguson, A.; Khan, U.; Walsh, M.; Lee, K.-Y.; Bismarck, A.; Shaffer, M.S.P.; Coleman, J.N.; Bergin, S.D. Understanding the Dispersion and Assembly of Bacterial Cellulose in Organic Solvents. Biomacromolecules 2016, 17, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Mathew, A.P.; Oksman, K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind. Crops Prod. 2012, 40, 232–238. [Google Scholar] [CrossRef]
- Gorgieva, S.; Štrancar, J.; Kokol, V. Evaluation of surface/interface-related physicochemical and microstructural properties of gelatin 3D scaffolds, and their influence on fibroblast growth and morphology. J. Biomed. Mater. Res. A 2014, 102, 3986–3997. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Yin, S.-H.; Li, S.-W.; Xie, F.; Zhang, L.-B.; Peng, J.-H. Study on the aqueous solution behavior and extraction mechanism of Nd(iii) in the presence of the complexing agent lactic acid with di-(2-ethylhexyl) phosphoric acid. RSC Adv. 2015, 5, 64550–64556. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Ikkala, O.; Berglund, L.A. Strong and Tough Cellulose Nanopaper with High Specific Surface Area and Porosity. Biomacromolecules 2011, 12, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.; Eronen, P.; Johansson, L.-S.; Malho, J.-M.; Ankerfors, M.; Lindström, T.; Ruokolainen, J.; Laine, J.; Österberg, M. The behaviour of cationic NanoFibrillar Cellulose in aqueous media. Cellulose 2011, 18, 1213–1226. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tammelin, T.; Schulfter, K.; Kiiskinen, H.; Samela, J.; Bismarck, A. High Performance Cellulose Nanocomposites: Comparing the Reinforcing Ability of Bacterial Cellulose and Nanofibrillated Cellulose. ACS Appl. Mater. Interfaces 2012, 4, 4078–4086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimpour, A.; Jahanshahi, M.; Mortazavian, N.; Madaeni, S.S.; Mansourpanah, Y. Preparation and characterization of asymmetric polyethersulfone and thin-film composite polyamide nanofiltration membranes for water softening. Appl. Surf. Sci. 2010, 256, 1657–1663. [Google Scholar] [CrossRef]
- Le Van Mao, R.; Vu, N.T.; Xiao, S.; Ramsaran, A. Modified zeolites for the removal of calcium and magnesium from hard water. J. Mater. Chem. 1994, 4, 1143–1147. [Google Scholar] [CrossRef]
- Katz, A.K.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium Ion Coordination: A Comparison with That of Beryllium, Magnesium, and Zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Bock, C.W.; Kaufman, A.; Glusker, J.P. Coordination of water to magnesium cations. Inorg. Chem. 1994, 33, 419–427. [Google Scholar] [CrossRef]
- Zachariah, Z.; Espinosa-Marzal, R.M.; Spencer, N.D.; Heuberger, M.P. Stepwise collapse of highly overlapping electrical double layers. Phys. Chem. Chem. Phys. 2016, 18, 24417–24427. [Google Scholar] [CrossRef] [Green Version]
- Millar, G.J.; Papworth, S.; Couperthwaite, S.J. Exploration of the fundamental equilibrium behaviour of calcium exchange with weak acid cation resins. Desalination 2014, 351, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Muqeet, M.; Khalique, A.; Qureshi, U.A.; Mahar, R.B.; Ahmed, F.; Khatri, Z.; Kim, I.S.; Brohi, K.M. Aqueous hardness removal by anionic functionalized electrospun cellulose nanofibers. Cellulose 2018, 25, 5985–5997. [Google Scholar] [CrossRef]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the Mineral Content of Tap Water and Bottled Waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Karnitz, O.; Gurgel, L.V.A.; Gil, L.F. Removal of Ca(II) and Mg(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse grafted with EDTA dianhydride (EDTAD). Carbohydr. Polym. 2010, 79, 184–191. [Google Scholar] [CrossRef]








| Grammage (gsm) | Adsorption Capacity (mg g−1) | Adsorption per Unit Area (mg m−2) | ||
|---|---|---|---|---|
| Ca2+ | Mg2+ | Ca2+ | Mg2+ | |
| 5 | 24.5 | - | 150 | - |
| 10 | 21.2 | - | 230 | - |
| 20 | 17.4 | - | 330 | - |
| Grammage (gsm) | Adsorption Capacity (mg g−1) | Adsorption Capacity (mmol g−1) | Adsorption per Unit Area (mg m−2) | |||
|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Ca2+ | Mg2+ | Ca2+ | Mg2+ | |
| 3 | 90.8 | 68.9 | 2.26 | 2.84 | 270 | 210 |
| 9 | 35.7 | 25.1 | 0.89 | 1.03 | 320 | 230 |
| 14 | 22.6 | 12.3 | 0.56 | 0.51 | 320 | 180 |
| Material | Reference | Adsorption Capacity (mg g−1) | |
|---|---|---|---|
| Ca2+ | Mg2+ | ||
| Phosphorylated TEMPO-CNF | this study | 91 | 69 |
| Mesoporous LTA zeolite | [7] | 108 | 28 |
| Carboxylated polyacrylic resin | [63] | 51 | - |
| Carbon nanotube sheets | [2] | 80 | 94 |
| Electrospun anionic cellulose nanofibers | [64] | 58 | 66 |
| Sugarcane bagasse | [66] | 14 | 16 |
| EDTA dianhydride grafted sugarcane bagasse | [66] | 46 | 23 |
| Natural pumice | [15] | 57 | 45 |
| Modified pumice | [15] | 62 | 56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, A.; Kobkeatthawin, T.; Mayer, F.; Plessl, C.; Gorgieva, S.; Kokol, V.; Bismarck, A. Rapid Water Softening with TEMPO-Oxidized/Phosphorylated Nanopapers. Nanomaterials 2019, 9, 136. https://doi.org/10.3390/nano9020136
Mautner A, Kobkeatthawin T, Mayer F, Plessl C, Gorgieva S, Kokol V, Bismarck A. Rapid Water Softening with TEMPO-Oxidized/Phosphorylated Nanopapers. Nanomaterials. 2019; 9(2):136. https://doi.org/10.3390/nano9020136
Chicago/Turabian StyleMautner, Andreas, Thawanrat Kobkeatthawin, Florian Mayer, Christof Plessl, Selestina Gorgieva, Vanja Kokol, and Alexander Bismarck. 2019. "Rapid Water Softening with TEMPO-Oxidized/Phosphorylated Nanopapers" Nanomaterials 9, no. 2: 136. https://doi.org/10.3390/nano9020136
APA StyleMautner, A., Kobkeatthawin, T., Mayer, F., Plessl, C., Gorgieva, S., Kokol, V., & Bismarck, A. (2019). Rapid Water Softening with TEMPO-Oxidized/Phosphorylated Nanopapers. Nanomaterials, 9(2), 136. https://doi.org/10.3390/nano9020136







