Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material
Abstract
:1. Introduction
2. Experimental
2.1. Materials
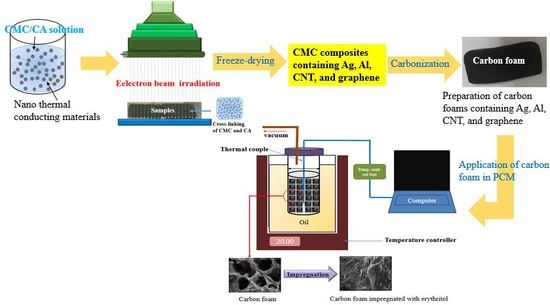
2.2. Preparation of Simple CMC Carbon Foam and CMC Carbon Foams Containing Nano Thermal Conducting Materials
2.3. Impregnation of Erythritol into Carbon Foam and Carbon Foams Containing Various Nano Thermal Conducting Materials and Its Characterization
3. Results and discussion
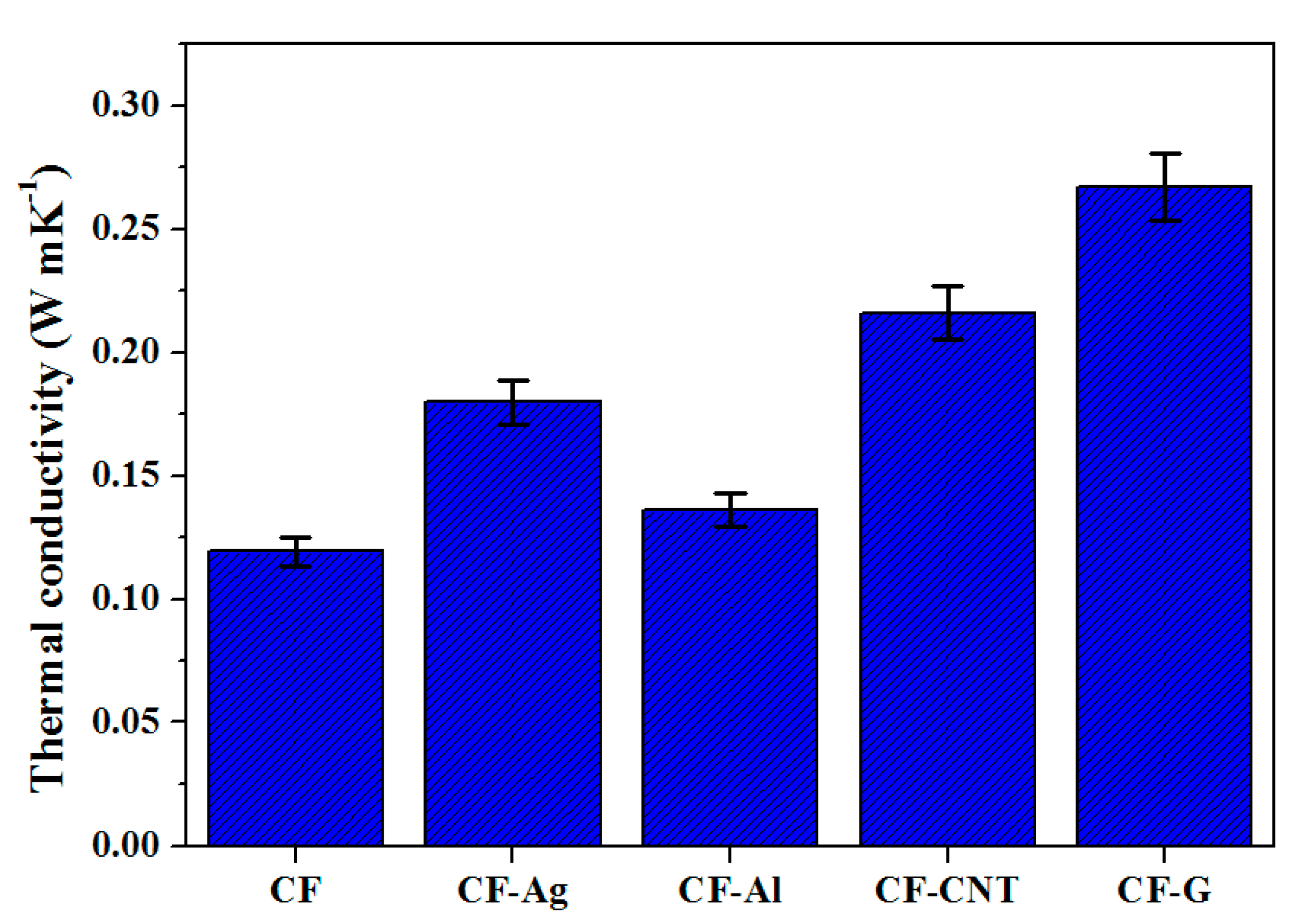
3.1. Thermal Conductivity of the Various Carbon Foams
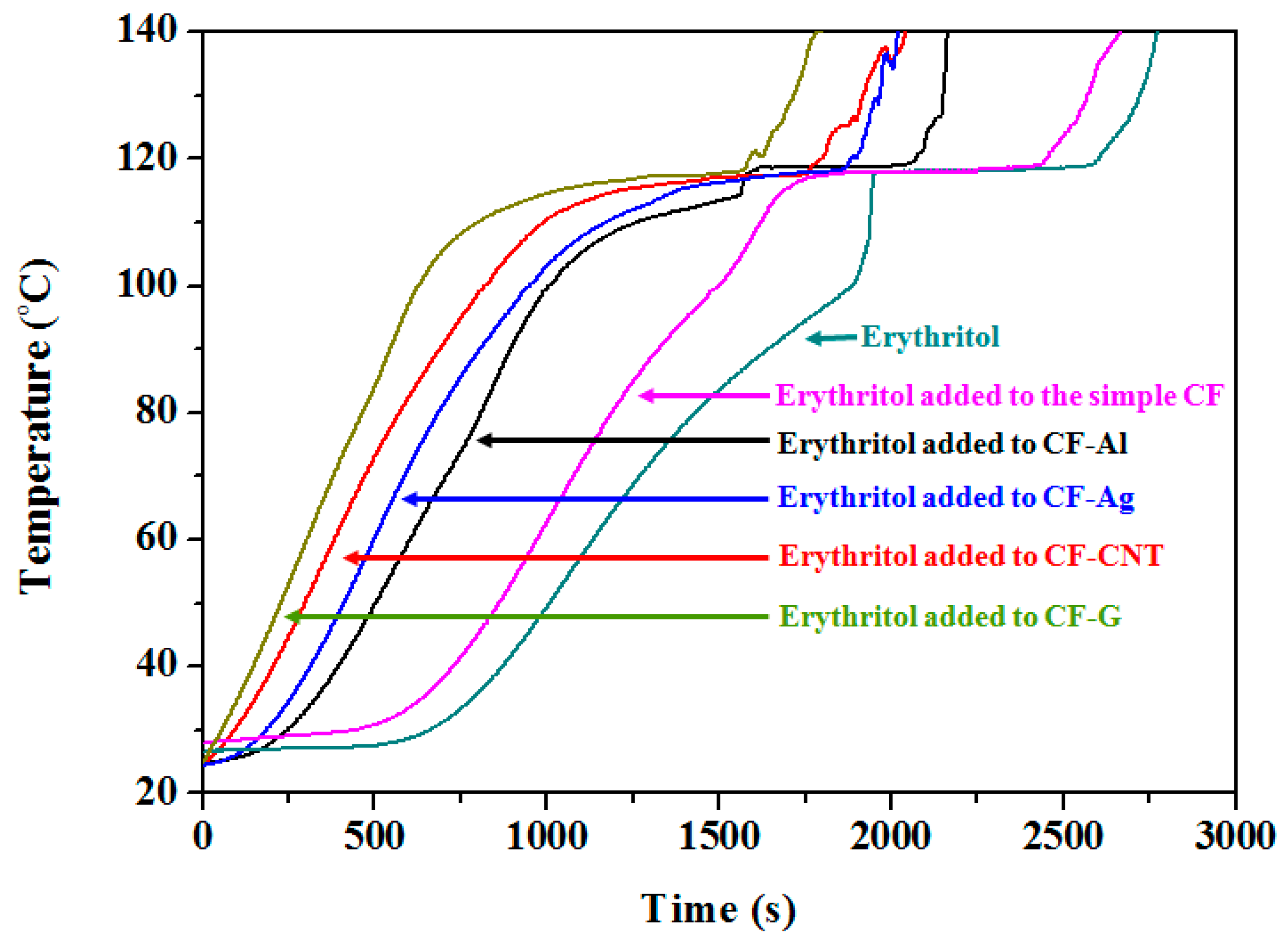
3.2. Effects of Carbon Foams Containing Nano Thermal Conducting Materials on the Melting of Erythritol
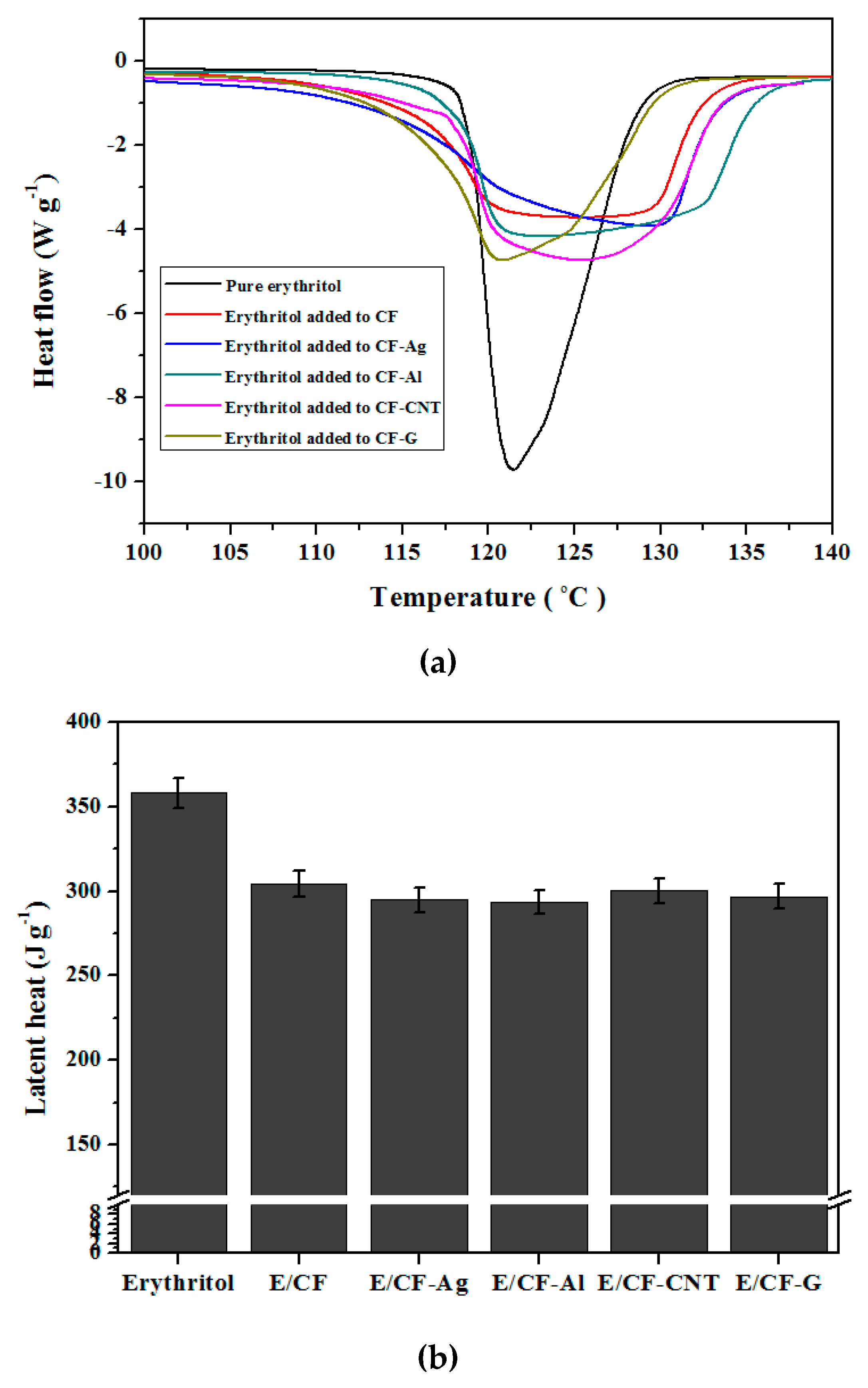
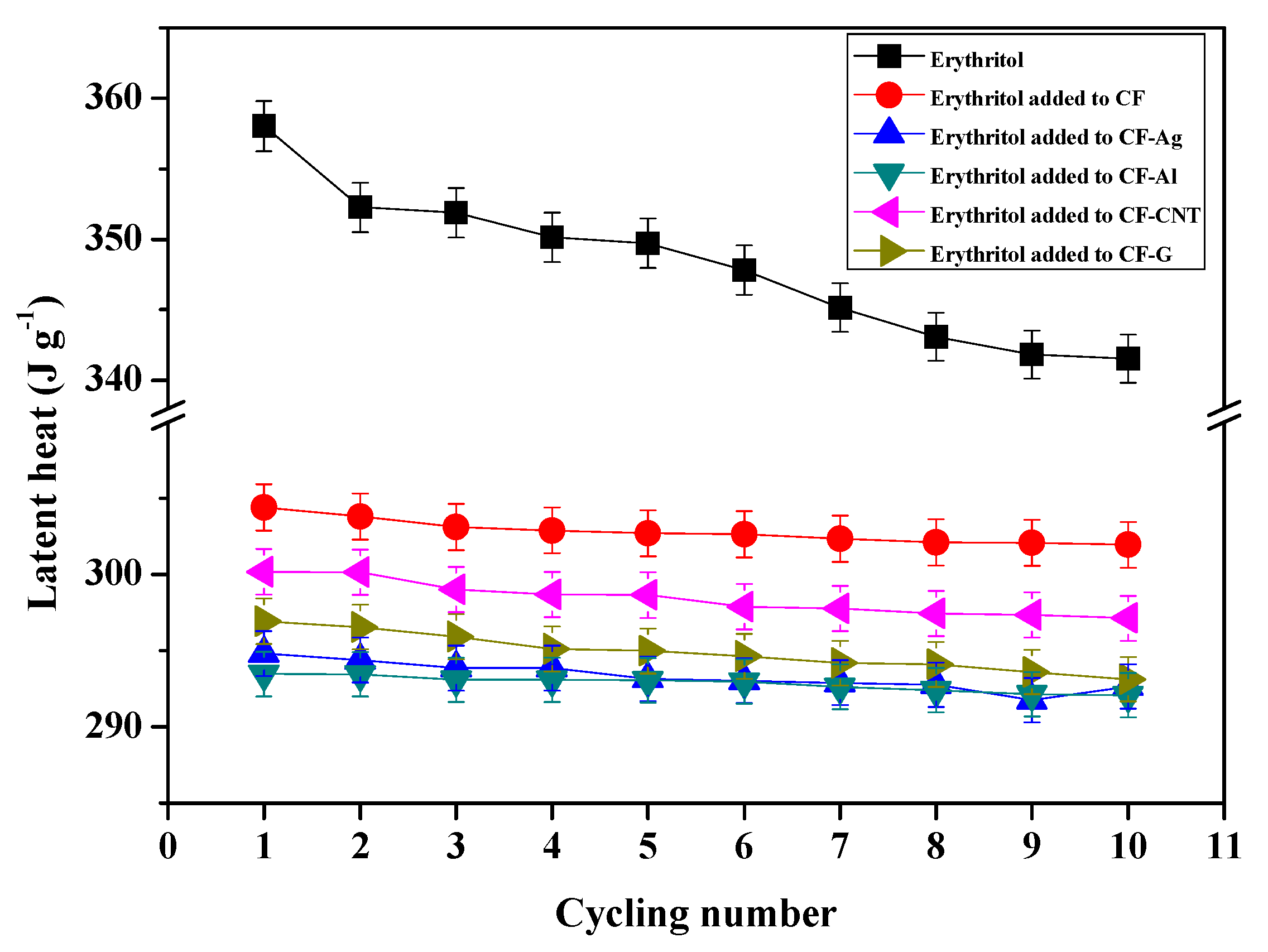
3.3. Thermal Effects of Simple Carbon Foam and Carbon Foams Containing Various Nano Thermal Conducting Materials on the Latent Heat Storage of Erythritol
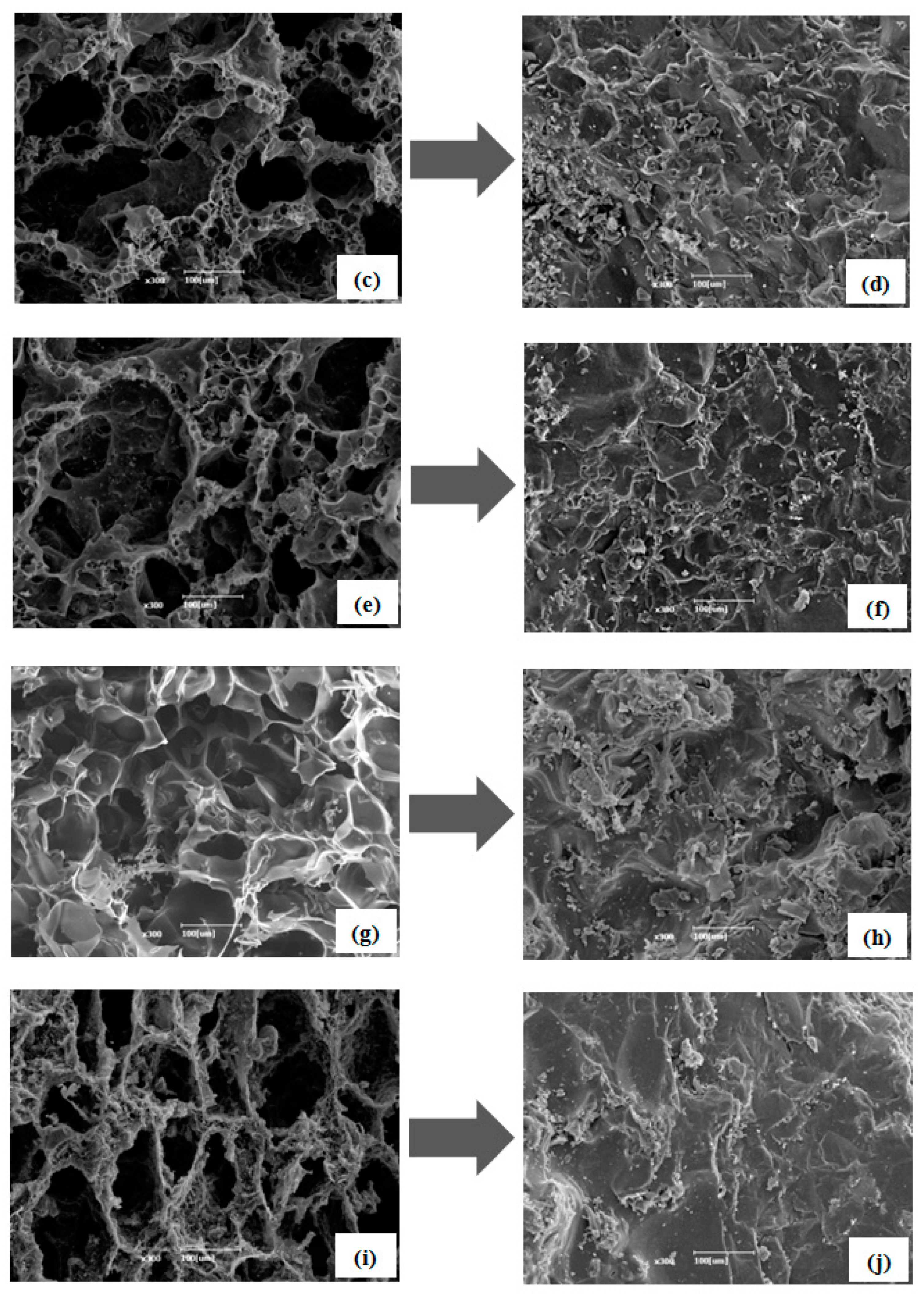
3.4. Scanning Electron Microscopy (SEM) Images for the Impregnation of Erythritol into Carbon Foams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haillot, D.; Bauer, T.; Tamme, R. Thermal analysis of phase change materials in the temperature range 120–150 °C. Themochim. Acta 2011, 513, 49–59. [Google Scholar] [CrossRef]
- Finck, C.; Li, R.L.; Kramer, R.; Zeirler, W. Quantifying demand flexibility of power-to-heat and thermal energy storage in the control of building heating systems. Appl. Energy 2018, 209, 409–425. [Google Scholar] [CrossRef]
- Kjaizawa, A.; Kamano, H.; Kawai, A.; Jozuka, T.; Senda, T.; Maruoka, N.; Okinaka, N.; Akiyama, T. Tchnical feasibility study of waste heat transportation system using phase change material from industry to city. ISIJ Int. 2008, 48, 540–548. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Waste heat transportation system, using phase change material (PCM) from steelworks to chemical plant. Resour. Conserv. Recycl. 2010, 54, 1000–1006. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2011, 15, 112–130. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Jensen, S.Ø.; Marszal-Pomianowska, A.; Lollini, R.; Pasut, W.; Knotzer, A.; Engelmann, P.; Stafford, A.; Reyndersm, G. IEA EBC annex 67 energy flexible buildings. Energy Build. 2017, 155, 25–34. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Shin, H.K.; Rhee, K.Y.; Park, S.J. Effects of exfoliated graphite on the thermal properties of erythritol-based composites used as phase-change materials. Compos. Part B-Eng. 2016, 96, 350–353. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, H.K.; Park, M.; Rhee, K.Y.; Park, S.J. Thermal characterization of erythritol/expanded graphite composites for high thermal storage capacity. Carbon 2014, 68, 67–72. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Su, J.; Zhang, X.; Han, N.; Wang, X. Microstructure and thermal reliability of microcapsules containing phase change material with self-assembled graphene/organic nano-hybrid shells. Nanomaterials 2018, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.G.; Comuñas, M.J.P.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-based phase change materials nano-enhanced with functionalized graphene nanoplatelets. Nanomaterials 2017, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, X.; Zhang, C.; Huang, Y.; Chen, Y. Melting behaviors of PCM in porous metal foam charterized by fractal geometry. Int. J. Heat Mass Transf. 2017, 113, 1031–1042. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Ebadi, S.; Venkateshwar, K.; Tansnim, S.H.; Mahmud, S.; Dutta, A. Charging nanopartivle enhanced bio based PCM in open cell metallic foams: An experimental investigation. Appl. Therm. Eng. 2019, 148, 1029–1042. [Google Scholar] [CrossRef]
- Andreozzi, A.; Buonomo, B.; Ercole, D.; Manca, O. Solar energy latent thermal storage by phase change materials (PCMs) in a honeycomb system. Therm. Sci. Eng. Prog. 2018, 6, 410–420. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Deng, Y.; Guan, W.; Wang, X.; Qian, T. Preparation of paraffin/porous TiO2 foams with enhanced thermal conductivity as PCM, by covering the TiO2 surface with a carbon layer. Appl. Energy 2016, 171, 37–45. [Google Scholar] [CrossRef]
- Wu, Z.G.; Sheng, W.C.; Tao, W.Q.; Li, Z. A novel experimental-numerical method for studying the thermal behaviors of phase change material in a porous cavity. Sol. Energy 2018, 169, 325–334. [Google Scholar] [CrossRef]
- Xiao, H.; Tao, C.; Li, Y.; Chen, X.; Huang, J.; Wang, J. Dopamine assisted one-step pyrolysis of glucose for the preparation of porous carbon with a high surface area. Nanomaterials 2018, 8, 854. [Google Scholar] [CrossRef]
- Sagisaka, K.; Kondo, A.; Iiyama, T.; Kimura, M.; Fujimoto, H.; Touhara, H.; Hattori, Y. Hollow structured porous carbon fibers with the inherent texture of the cotton fibers. Chem. Phys. Lett. 2018, 710, 118–122. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, M.; Kim, H.Y.; Park, S.J. Thermal property and latent heat energy storage behavior of sodium acetate trihydrate composites containing expanded graphite and carboxymethyl cellulose for phase change materials. Appl. Therm. Eng. 2015, 75, 978–983. [Google Scholar] [CrossRef]
- Karthik, M.; Faik, A.; Blanco-Rodriguez, P.; Rodriguez-Aseguinolaza, J.; D’Auanno, B. Preparation of erythritol-graphite foam phase change composite with enhanced thermal conductivity for thermal energy storage applications. Carbon 2015, 94, 266–276. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Luo, Z.; Zeng, Y. Thermal performance enhancement of erythritol/carbon foam composites via surface modification of carbon foam. IOP Mater. Sci. Eng. 2017, 182, 012009. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Qu, J.; Han, Q.; Gao, F.; Qiu, J. Carbon foams produced from lignin-phenol-formaldehyde resin for oil/water separation. New Carbon Mater. 2017, 32, 86–91. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, B.; Hou, H.; Zhang, R. Mesophase pitch based carbon foams as sound absorbers. Mater. Lett. 2017, 212, 243–246. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Wu, W.; Liu, X.; Lin, X.; Shen, X.; Wang, Z.; Li, R.K.Y.; Yang, Z.; Lau, K.T.; et al. Graphene foam/carbon nanotube/poly(dimethyl siloxane) composites as excellent sound absorber. Compos. Part A Appl. Sci. Manuf. 2017, 102, 391–399. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, Y.S.; Kwac, L.K.; Chae, S.H.; Shin, H.K. Synthesis of carbon foam from waste artificial marble powder and carboxymethyl cellulose via electron beam irradiation and its characterization. Materials 2018, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Kwac, L.K.; Kim, Y.S.; Shin, H.K.; Rhee, K.Y. Synthesis and characterization of eco-friendly carboxymethyl cellulose based carbon foam using electron beam irradiation. Compos. Part B-Eng. 2018, 151, 154–160. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, M.; Kang, P.H.; Rhee, K.Y.; Park, S.J. Role of electron beam irradiation on superabsorbent behaviors of carboxymethyl cellulose. Res. Chem. Intermed. 2015, 41, 6815–6823. [Google Scholar] [CrossRef]
- Benslimane, A.; Bahlouli, I.M.; Bekkour, K.; Hammiche, D. Thermal gelation properties of carboxymethyl cellulose and bentonite-carboxymethyl cellulose dispersions: Rheo-logical considerations. Appl. Clay Sci. 2016, 132–133, 702–710. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Angrawal, P.R.; Kumar, R.; Teotia, S.; Kumari, S.; Mondal, D.P.; Dhakate, S.R. Lightweight, high electrical and thermal conducting carbon-rGO composites foam for superior electromagnetic interference shielding. Compos. Part B-Eng. 2019, 160, 131–139. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Pizzi, A.; Celzard, A. Thermal conductivity improvement of composite carbon foams based on tannin-based disordered carbon matrix and graphite fillers. Mater. Des. 2015, 83, 635–643. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.G.; Kim, Y.-S.; Kwac, L.K.; Shin, H.J.; Lee, S.O.; Lee, U.S.; Shin, H.K. Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material. Nanomaterials 2019, 9, 158. https://doi.org/10.3390/nano9020158
Kim HG, Kim Y-S, Kwac LK, Shin HJ, Lee SO, Lee US, Shin HK. Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material. Nanomaterials. 2019; 9(2):158. https://doi.org/10.3390/nano9020158
Chicago/Turabian StyleKim, Hong Gun, Yong-Sun Kim, Lee Ku Kwac, Hee Jae Shin, Sang Ok Lee, U Sang Lee, and Hye Kyoung Shin. 2019. "Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material" Nanomaterials 9, no. 2: 158. https://doi.org/10.3390/nano9020158
APA StyleKim, H. G., Kim, Y.-S., Kwac, L. K., Shin, H. J., Lee, S. O., Lee, U. S., & Shin, H. K. (2019). Latent Heat Storage and Thermal Efficacy of Carboxymethyl Cellulose Carbon Foams Containing Ag, Al, Carbon Nanotubes, and Graphene in a Phase Change Material. Nanomaterials, 9(2), 158. https://doi.org/10.3390/nano9020158