Highly Controllable Synthesis and DFT Calculations of Double/Triple-Halide CsPbX3 (X = Cl, Br, I) Perovskite Quantum Dots: Application to Light-Emitting Diodes
Abstract
:1. Introduction
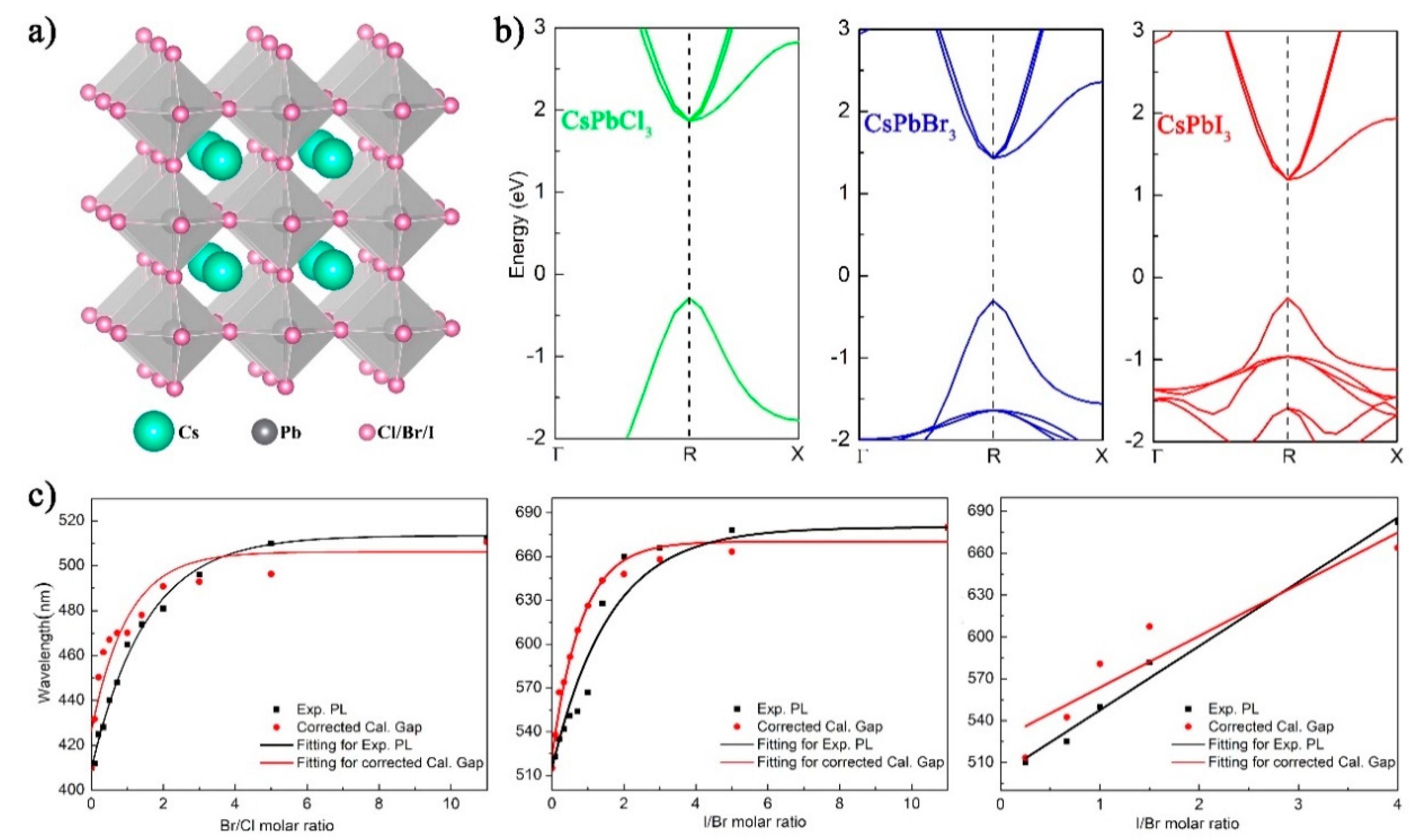
2. Results and Discussion
3. Conclusions
4. Experimental Section
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.V.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J.; et al. Prospects of Nanoscience with Nanocrystals. Acs Nano 2015, 9, 1012–1057. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lee, Y.-L. Chemical bath deposition of CdS quantum dots onto mesoscopic films for application in quantum-dot-sensitized solar cells. Appl. Phys. Lett. 2007, 91, 053503. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, C.-Y.; Liu, C.-W.; Chang, H.-T. Electrocatalytic sulfur electrodes for CdS/CdSe quantum dot-sensitized solar cells. Chem. Commun. 2010, 46, 5485–5487. [Google Scholar] [CrossRef] [PubMed]
- Chen, O.; Zhao, J.; Chauhan, V.P.; Cui, J.; Wong, C.; Harris, D.K.; Wei, H.; Han, H.-S.; Fukumura, D.; Jain, R.K.; et al. Compact high-quality CdSe–CdS core–shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat. Mater. 2013, 12, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Wang, W.; Zhang, J.; Xu, B.; Karen, K.L.; Zheng, Y.; Liu, S.; Chen, S.; Wang, K.; et al. Hybrid Perovskite Light-Emitting Diodes Based on Perovskite Nanocrystals with Organic–Inorganic Mixed Cations. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 15424–15428. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, X.; Xiao, L.; Zeng, H.; Sun, H. Nonlinear Absorption and Low-Threshold Multiphoton Pumped Stimulated Emission from All-Inorganic Perovskite Nanocrystals. Nano Lett. 2016, 16, 448–453. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Citation for: Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals with Tunable Composition and Thickness by Ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal Synthesis of High-Quality All-Inorganic Cesium Lead Halide Perovskite Nanocrystals: From Nanocube to Ultrathin Nanowire. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.C.; Zou, Y.T.; Chen, M.; Wu, L.Z.; Yang, D.; Yuan, X.L.; Fan, J.; Sun, B.Q.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Beal, R.E.; Slotcavage, D.J.; Leijtens, T.; Bowring, A.R.; Belisle, R.A.; Nguyen, W.H.; Burkhard, G.F.; Hoke, E.T.; Mcgehee, M.D. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. J. Phys. Chem. Lett. 2016, 7, 746–751. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Zhang, Y.; Chen, Y.; Li, X.; Wu, D.; Xu, T.; Shan, C.; Du, G. High-Efficiency and Air-Stable Perovskite Quantum Dots Light-Emitting Diodes with an All-Inorganic Heterostructure. Nano Lett. 2017, 17, 313–321. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Li, J.; Xue, J.; Dong, Y.; Li, X.; Zeng, H. Monolayer and Few-Layer All-Inorganic Perovskites as a New Family of Two-Dimensional Semiconductors for Printable Optoelectronic Devices. Adv. Mater. 2016, 28, 4861–4869. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, B.; Lee, S.H.; Lee, M.S.; Lee, J.S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2015, 52, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Cui, Y.; Deng, H.X.; Huang, L.; Wei, Z.M.; Li, J.B. Modulation of electronic and optical properties in mixed halide perovskites CsPbCl3xBr3(1−x) and CsPbBr3xI3(1−x). Appl. Phys. Lett. 2017, 110, 113901. [Google Scholar] [CrossRef]
- Yin, W.J.; Yan, Y.F.; Wei, S.H. Anomalous Alloy Properties in Mixed Halide Perovskites. J. Phys. Chem. Lett. 2014, 5, 3625–3631. [Google Scholar] [CrossRef]



| Percentage of Cl | Percentage of Br | Cl:Br Molar Ratio | ΔH (eV) | |
|---|---|---|---|---|
| 1 | 8.3% | 91.7% | 1:11 | 0.041 |
| 2 | 16.7% | 83.3% | 2:10 | 0.035 |
| 3 | 25% | 75% | 3:9 | 0.142 |
| 4 | 33.3% | 66.7% | 4:8 | 0.071 |
| 5 | 41.7% | 58.3% | 5:7 | 0.092 |
| 6 | 50% | 50% | 6:6 | 0.186 |
| 7 | 58.3% | 41.7% | 7:5 | 0.108 |
| 8 | 66.7% | 33.3% | 8:4 | 0.050 |
| 9 | 75% | 25% | 9:3 | 0.067 |
| 10 | 83.3% | 16.7% | 10:2 | 0.067 |
| 11 | 91.7% | 8.3% | 11:1 | 0.071 |
| Percentage of Br | Percentage of I | Br:I Molar Ratio | ΔH (eV) | |
|---|---|---|---|---|
| 1 | 8.3% | 91.7% | 1:11 | 0.041 |
| 2 | 16.7% | 83.3% | 2:10 | 0.035 |
| 3 | 25% | 75% | 3:9 | 0.142 |
| 4 | 33.3% | 66.7% | 4:8 | 0.071 |
| 5 | 41.7% | 58.3% | 5:7 | 0.092 |
| 6 | 50% | 50% | 6:6 | 0.186 |
| 7 | 58.3% | 41.7% | 7:5 | 0.108 |
| 8 | 66.7% | 33.3% | 8:4 | 0.050 |
| 9 | 75% | 25% | 9:3 | 0.067 |
| 10 | 83.3% | 16.7% | 10:2 | 0.067 |
| 11 | 91.7% | 8.3% | 11:1 | 0.071 |
| Cl:Br:I Molar Ratio | ΔH (eV) | |
|---|---|---|
| 1 | 2:8:2 | 0.129 |
| 2 | 2:6:4 | 0.185 |
| 3 | 2:5:5 | 0.275 |
| 4 | 2:4:6 | 0.252 |
| 5 | 2:2:8 | 0.214 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, X.; Li, X.; Ågren, H.; Chen, G. Highly Controllable Synthesis and DFT Calculations of Double/Triple-Halide CsPbX3 (X = Cl, Br, I) Perovskite Quantum Dots: Application to Light-Emitting Diodes. Nanomaterials 2019, 9, 172. https://doi.org/10.3390/nano9020172
Lao X, Li X, Ågren H, Chen G. Highly Controllable Synthesis and DFT Calculations of Double/Triple-Halide CsPbX3 (X = Cl, Br, I) Perovskite Quantum Dots: Application to Light-Emitting Diodes. Nanomaterials. 2019; 9(2):172. https://doi.org/10.3390/nano9020172
Chicago/Turabian StyleLao, Xinyue, Xiyu Li, Hans Ågren, and Guanying Chen. 2019. "Highly Controllable Synthesis and DFT Calculations of Double/Triple-Halide CsPbX3 (X = Cl, Br, I) Perovskite Quantum Dots: Application to Light-Emitting Diodes" Nanomaterials 9, no. 2: 172. https://doi.org/10.3390/nano9020172






