Novel Superhydrophobic Sand and Polyurethane Sponge Coated with Silica/Modified Asphaltene Nanoparticles for Rapid Oil Spill Cleanup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.3. Characterization of HSNPs
2.4. Surface Modification for Superhydrophobic Sand
2.5. Efficiency of the Superhydrophobic Sand and Hydrophobic PU Sponge in the Collection of Oil Spills
3. Results
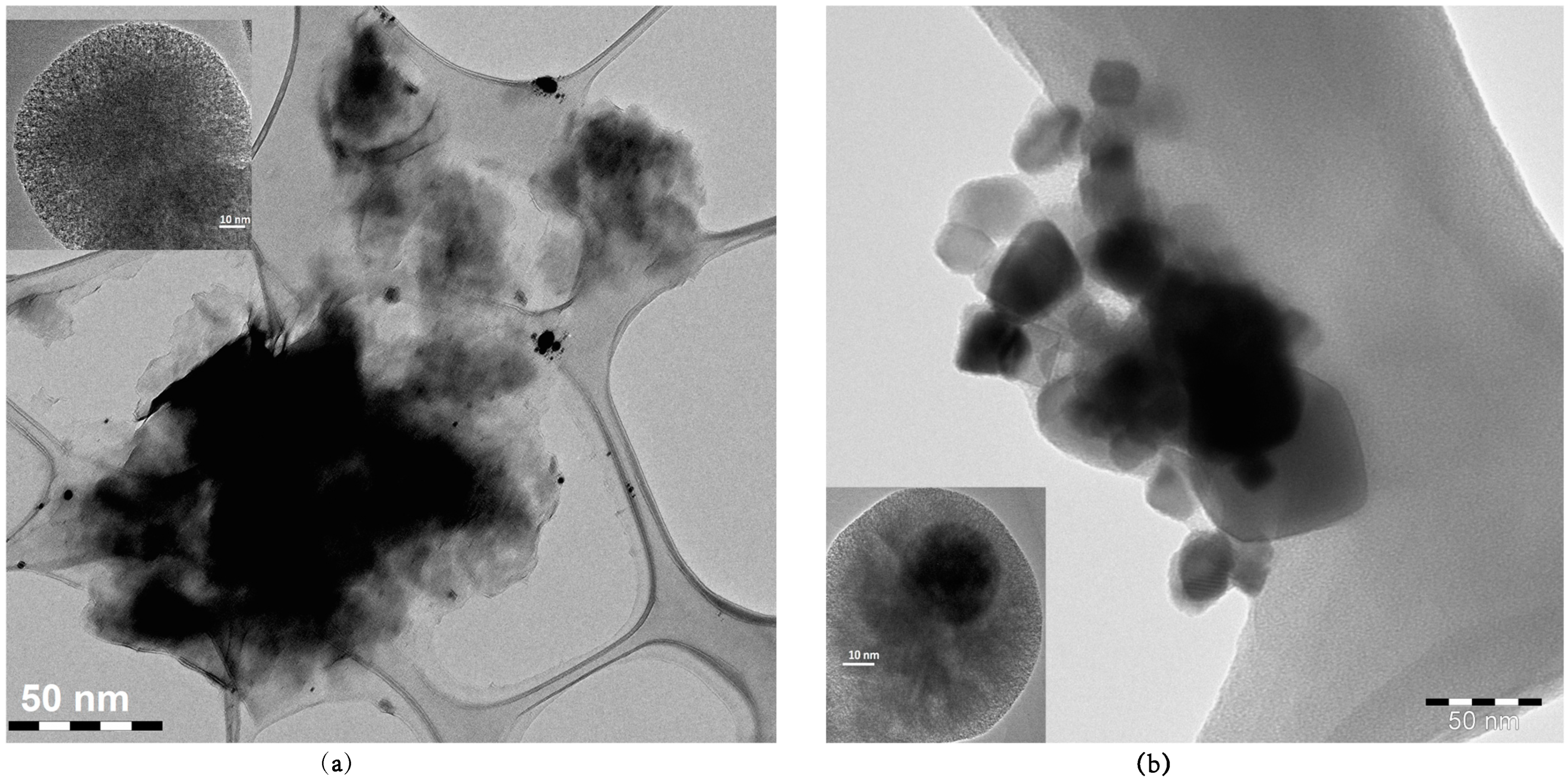
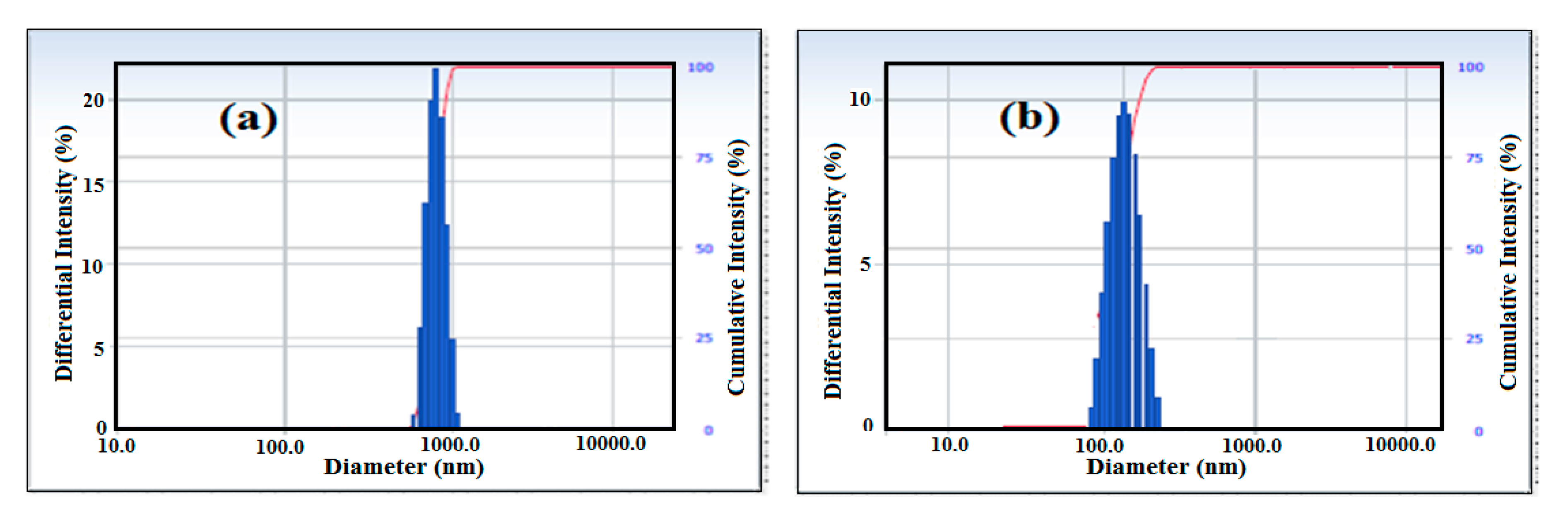
3.1. Characterization of HSNPs
3.2. Wetting Characteristics of HSNPs
3.3. Efficiency of the Treated Superhydrophobic Sand and PU Sponge as Oil Spill Collectors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kingston, P.F. Long-term environmental impact of oil spills. Spill Sci. Technol. Bull. 2002, 7, 53–61. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.; Hoang, N.; Tan, V.B.; Duong, H.M. Cellulose aerogel from paper waste for crude oil spill cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Nyankson, E.; Rodene, D.; Gupta, R.B. Advancements in crude oil spill remediation research after the Deepwater Horizon oil spill. Water Air Soil Pollut. 2016, 227, 29. [Google Scholar] [CrossRef]
- Atta, A.M.; El-Ghazawy, R.A.; Farag, R.K.; Abdel-Azim, A.-A.A. Crosslinked reactive macromonomers based on polyisobutylene and octadecyl acrylate copolymers as crude oil sorbers. React. Funct. Polym. 2006, 66, 931–943. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M.; ElSaeed, S.M. Application of new amphiphilic ionic liquid based on ethoxylated octadecylammonium tosylate as demulsifier and petroleum crude oil spill dispersant. J. Ind. Eng. Chem. 2016, 33, 122–130. [Google Scholar] [CrossRef]
- Abdullah, M.; Atta, A.; Allohedan, H.; Alkhathlan, H.; Khan, M.; Ezzat, A. Green synthesis of hydrophobic magnetite nanoparticles coated with plant extract and their application as petroleum oil spill collectors. Nanomaterials 2018, 8, 855. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.; Al-Lohedan, H.A.; Atta, A.M. Novel magnetic iron oxide nanoparticles coated with sulfonated asphaltene as crude oil spill collectors. RSC Adv. 2016, 6, 59242–59249. [Google Scholar] [CrossRef]
- Atta, A.M.; Brostow, W.; Lobland, H.E.H.; Hasan, A.-R.M.; Perez, J.M. Porous polymer oil sorbents based on PET fibers with crosslinked copolymer coatings. RSC Adv. 2013, 3, 25849–25857. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; Hashem, A.I. Synthesis and application of monodisperse hydrophobic magnetite nanoparticles as an oil spill collector using an ionic liquid. RSC Adv. 2017, 7, 16524–16530. [Google Scholar] [CrossRef] [Green Version]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Synthesis of reusable silicone foam containing carbon nanotubes for oil spill remediation. J. Appl. Polym. Sci. 2018, 135, 46067. [Google Scholar] [CrossRef]
- Peng, H.; Wang, H.; Wu, J.; Meng, G.; Wang, Y.; Shi, Y.; Liu, Z.; Guo, X. Preparation of superhydrophobic magnetic cellulose sponge for removing oil from water. Ind. Eng. Chem. Res. 2016, 55, 832–838. [Google Scholar] [CrossRef]
- Khosravi, M.; Azizian, S. Synthesis of a novel highly oleophilic and highly hydrophobic sponge for rapid oil spill cleanup. ACS Appl. Mater. Interfaces 2015, 7, 25326–25333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Xu, X.; Men, X.; Zhu, X. Facile fabrication of superhydrophobic sponge with selective absorption and collection of oil from water. Ind. Eng. Chem. Res. 2013, 52, 9411–9416. [Google Scholar] [CrossRef]
- Chen, X.; Weibel, J.A.; Garimella, S.V. Continuous oil–water separation using polydimethylsiloxane-functionalized melamine sponge. Ind. Eng. Chem. Res. 2016, 55, 3596–3602. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, W.; Yu, Y.; Hou, H.; Zhu, Z. One-Step Preparation of Highly Hydrophobic and oleophilic melamine sponges via metal-ion-induced wettability transition. ACS Appl. Mater. Interfaces 2018, 10, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Prendergast, D.P.; MacFarlane, J.; Bagatin, R.; Stellacci, F.; Gschwend, P.M. Hydrophobic meshes for oil spill recovery devices. ACS Appl. Mater. Interfaces 2013, 5, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, X.; Ouyang, X.; Wen, C. Flexible Superhydrophobic and Superoleophilic MoS2 sponge for highly efficient oil-water separation. Sci. Rep. 2016, 6, 27207. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Liu, T.; Xu, G.; Chen, G.; Li, H.; Liu, L.; Zhuo, Q.; Zhang, J.; Yan, C. Superhydrophobic hBN-regulated sponges with excellent absorbency fabricated using a green and facile method. Sci. Rep. 2017, 7, 45065. [Google Scholar] [CrossRef]
- Tu, C.W.; Tsai, C.H.; Wang, C.F.; Kuo, S.W.; Chang, F.C. Fabrication of superhydrophobic and superoleophilic polystyrene surfaces by a facile one-step method. Macromol. Rapid Commun. 2007, 28, 2262–2266. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Qasim, K.; Xia, J.; Lei, W.; Wang, B.P. A hierarchical mesh film with superhydrophobic and superoleophilic properties for oil and water separation. J. Chem. Technol. Biotechnol. 2012, 87, 427–430. [Google Scholar] [CrossRef]
- Lee, C.; Baik, S. Vertically-aligned carbon nano-tube membrane filters with superhydrophobicity and superoleophilicity. Carbon 2010, 48, 2192–2197. [Google Scholar] [CrossRef]
- Lee, C.H.; Johnson, N.; Drelich, J.; Yap, Y.K. The performance of superhydrophobic and superoleophilic carbon nanotube meshes in water–oil filtration. Carbon 2011, 49, 669–676. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Lu, Q. Creating superhydrophobic surfaces with flowery structures on nickel substrates through a wet-chemical-process. J. Mater. Chem. 2007, 17, 4772–4776. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, Y.; Jiang, L. Microscale and nanoscale hierarchical structured mesh films with superhydrophobic and superoleophilic properties induced by long-chain fatty acids. Nanotechnology 2006, 18, 015103. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, M.; Wang, H. Separating small amount of water and hydrophobic solvents by novel superhydrophobic copper meshes. Appl. Surf. Sci 2008, 254, 6002–6006. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Amphiphile grafted membranes for the separation of oil-in-water dispersions. J. Colloid Interface Sci. 2009, 329, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Xu, X.; Zhu, X.; Men, X.; Zhou, X. Superhydrophilic–superoleophobic coatings. J. Mater. Chem. 2012, 22, 2834–2837. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, X.; Akbulut, O.; Hu, J.; Suib, S.L.; Kong, J.; Stellacci, F. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 2008, 3, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, L. Metallic surfaces with special wettability. Nanoscale 2011, 3, 825–838. [Google Scholar] [CrossRef]
- Boglaienko, D.; Tansel, B. Instantaneous Stabilization of floating oils by surface application of natural granular materials (Beach sand and limestone). Mar. Pollut. Bull. 2015, 91, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Shirokoff, W.J.; Siddiqui, N.M.; Ali, F.M. Characterization of the Structure of Saudi Crude Asphaltenes by X-ray Diffraction. Energy Fuel 1997, 11, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, S.M.; Zhong, K.X.; Firoozabadi, A. Acid–base chemistry enables reversible colloid-to-solution transition of asphaltenes in non-polar systems. Soft Matter 2012, 8, 8778–8785. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, F.; Chen, Z. Reaction of asphalt and maleic anhydride: Kinetics and mechanism. Chem. Eng. J. 2010, 164, 230–237. [Google Scholar] [CrossRef]
- Boucher, J.; Wang, I.; Romine, R. Addition chemistry in asphalt. Am. Chem. Soc. Div. Pet. Chem. Prepr. (U. S.) 1990, 35, 556–561. [Google Scholar]
- León-Bermúdez, A.-Y.; Salazar, R. Synthesis and characterization of the polystyrene-asphaltene graft copolymer by FT-IR spectroscopy. CT F Ciencia Tecnología y Futuro 2008, 3, 158–167. [Google Scholar]
- Kong, L.; Uedono, A.; Smith, S.V.; Yamashita, Y.; Chironi, I. Synthesis of silica nanoparticles using oil-in-water emulsion and the porosity analysis. J. Sol-Gel Sci. Technol. 2012, 64, 309–314. [Google Scholar] [CrossRef]
- Tellez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J. Synthesis of inorganic-organic hybrid materials from TEOS, TBT and PDMS. J. Mater. Sci. 2003, 38, 1773–1780. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Gaikwad, R.; Hande, A.; Das, S.; Mitra, S.K.; Thundat, T. Determination of charge on asphaltene nanoaggregates in air using electrostatic force microscopy. Langmuir 2015, 31, 679–684. [Google Scholar] [CrossRef]
- Men, X.; Ge, B.; Li, P.; Zhu, X.; Shi, X.; Zhang, Z. Facile fabrication of superhydrophobic sand: Potential advantages for practical application in oil–water separation. J. Taiwan Inst. Chem. Eng. 2016, 60, 651–655. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, D.; Zhu, W.; Sathasivam, S.; Parkin, I.P. Facile fabrication of durable superhydrophobic SiO 2/polyurethane composite sponge for continuous separation of oil from water. RSC Adv. 2017, 7, 11362–11366. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]








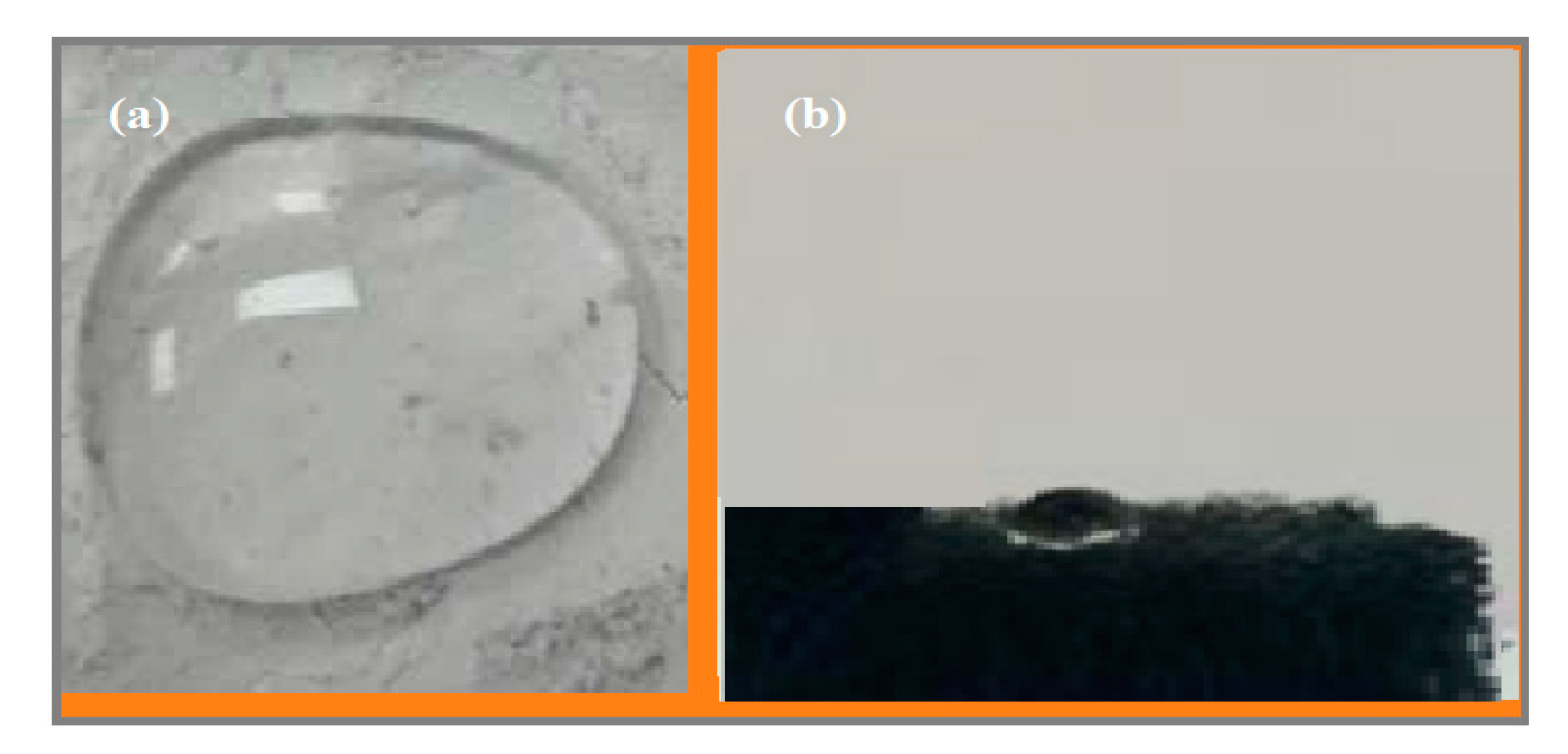

| HSNP-2 (ASAS wt.%) | Contact Angle (degree) |
|---|---|
| 40 | 170 ± 4 |
| 20 | 160 ± 3 |
| 10 | 155 ± 4 |
| 5 | 140 ± 2 |
| Ratio of HSNP-2: Sand (Wt.:Wt.) | Ratio of Sand:Crude oil (Wt.:Wt.) | Efficiency (CE%) |
|---|---|---|
| (1:2) | 1:1 | 92 ± 1 |
| 1:2 | 90 ± 2 | |
| 1:10 | 80 ± 3 | |
| (1:4) | 1:1 | 89 ± 3 |
| 1:2 | 85 ± 4 | |
| 1:10 | 58 ± 5 | |
| (1:8) | 1:1 | 78 ± 3 |
| 1:2 | 55 ± 4 | |
| 1:10 | 28 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atta, A.M.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Mohamed, N.H. Novel Superhydrophobic Sand and Polyurethane Sponge Coated with Silica/Modified Asphaltene Nanoparticles for Rapid Oil Spill Cleanup. Nanomaterials 2019, 9, 187. https://doi.org/10.3390/nano9020187
Atta AM, Abdullah MMS, Al-Lohedan HA, Mohamed NH. Novel Superhydrophobic Sand and Polyurethane Sponge Coated with Silica/Modified Asphaltene Nanoparticles for Rapid Oil Spill Cleanup. Nanomaterials. 2019; 9(2):187. https://doi.org/10.3390/nano9020187
Chicago/Turabian StyleAtta, Ayman M., Mahmood M. S. Abdullah, Hamad A. Al-Lohedan, and Nermen H. Mohamed. 2019. "Novel Superhydrophobic Sand and Polyurethane Sponge Coated with Silica/Modified Asphaltene Nanoparticles for Rapid Oil Spill Cleanup" Nanomaterials 9, no. 2: 187. https://doi.org/10.3390/nano9020187





