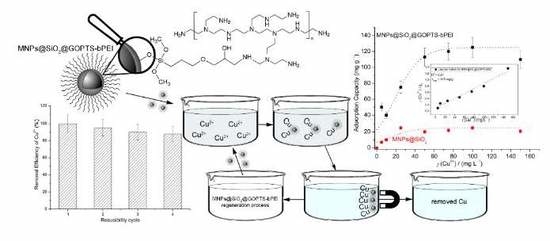
Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals:
2.2. Preparation of γ-Fe2O3 (MNPs) and SiO2-coated MNPs (MNPs@SiO2)
2.3. Systematic Covalent Linkage of GOPTS and bPEI (GOPTS-bPEI) onto MNPs@SiO2 (i.e., MNPs@SiO2@GOPTS-bPEI)
2.4. Characterization of the Nanoadsorbent
2.5. Cu2+ Adsorption Experiments
3. Results and Discussion
3.1. Nanoadsorbent Characterization
3.2. Effect of the Introduced bPEI onto Cu2+ Removal Efficiency
3.2.1. Effect of pH
3.2.2. Effect of Initial Cu2+ Concentration
3.2.3. Reusability Cycles for Regeneration Studies
3.3. Proposed Possible Adsorption Mechanism for Cu
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12. [Google Scholar]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Fawy, H.; Abdel-Hady, E. Study of sewage sludge use in agriculture and its effect on plant and soil. Agric. Biol. J. N. Am. 2010, 1, 1044–1049. [Google Scholar] [CrossRef]
- Bratina, B.; Šorgo, A.; Kramberger, J.; Ajdnik, U.; Zemljič, L.F.; Ekart, J.; Šafarič, R. From municipal/industrial wastewater sludge and FOG to fertilizer: A proposal for economic sustainable sludge management. J. Environ. Manag. 2016, 183, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- Pradel, M.; Aissani, L.; Villot, J.; Baudez, J.C.; Laforest, V. From waste to added value product: Towards a paradigm shift in life cycle assessment applied to wastewater sludge—A review. J. Clean. Prod. 2016, 131, 60–75. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, S.; Yu, H.; Zhang, T.; Cheng, H.; Sun, Q.; Xiao, R. Environmental risk assessment of pyrometallurgical residues derived from electroplating and pickling sludges. J. Clean. Prod. 2018, 177, 699–707. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2007, 1, 2661–2667. [Google Scholar] [CrossRef]
- Mudhoo, A.; Garg, V.K.; Wang, S. Removal of heavy metals by biosorption. Environ. Chem. Lett. 2012, 10, 109–117. [Google Scholar] [CrossRef]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Ji, Y.; Ma, H.; Song, Y.; He, B.; Li, J. A novel route for the removal of Cu(II) and Ni(II) ions via homogeneous adsorption by chitosan solution. J. Clean. Prod. 2018, 192, 801–808. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, J.H.; Shinkai, S. Functionalized magnetic nanoparticles as chemosensors and adsorbents for toxic metal ions in environmental and biological fields. Chem. Soc. Rev. 2011, 40, 4464. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Reactions, Occurrences and Uses, 2nd ed.; WILEY-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Kralj, S.; Makovec, D.; Čampelj, S.; Drofenik, M. Producing ultra-thin silica coatings on iron-oxide nanoparticles to improve their surface reactivity. J. Magn. Magn. Mater. 2010, 322, 1847–1853. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; John Wiley&Sons: New York, NY, USA, 1979; ISBN 047102404X. [Google Scholar]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Sun, W. Functionalization of surfaces with branched polymers. RSC Adv. 2016, 6, 42089–42108. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.; Berenguer-Murcia, Á; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar]
- Kim, C.; Lee, S.S.; Lafferty, B.J.; Giammar, D.E.; Fortner, J.D. Engineered superparamagnetic nanomaterials for arsenic (v) and chromium (vi) sorption and separation: Quantifying the role of organic surface coatings. Environ. Sci. Nano 2018, 5, 556–563. [Google Scholar] [CrossRef]
- Goon, I.Y.; Zhang, C.; Lim, M.; Gooding, J.J.; Amal, R. Controlled fabrication of polyethylenimine-functionalized magnetic nanoparticles for the sequestration and quantification of free Cu2+. Langmuir 2010, 26, 12247–12252. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, D.; Wei, Q.; Wei, D.; Yan, T.; Yan, L.; Hu, L.; Du, B. Rapid removal of Pb (II) from aqueous solution using branched polyethylenimine enhanced magnetic carboxymethyl chitosan optimized with response surface methodology. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, B.; Wang, H.; Zhao, F.; Chen, Y.; Sun, Q. Facile crosslinking synthesis of hyperbranch-substrate nanonetwork magnetite nanocomposite for the fast and highly efficient removal of lead ions and anionic dyes from aqueous solutions. RSC Adv. 2016, 6, 67057–67071. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Long, X.; Zhang, X.; Pan, B.; Qian, J. Multi-functional magnetic water purifier for disinfection and removal of dyes and metal ions with superior reusability. J. Hazard. Mater. 2018, 347, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Aishah, N.; Amin, S. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Hermanson, T.G. Bioconjugate Techniques, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705013. [Google Scholar]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid materials: Magnetite-Polyethylenimine-Montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, M.; Xu, J.; Lu, A.; Shi, Y. Magnetic Fe3O4 Nanoparticles Modified With Polyethyleneimine for the Removal of Pb(II). Clean Soil Air Water 2016, 44, 1–8. [Google Scholar] [CrossRef]
- Sui, N.; Wang, L.; Wu, X.; Li, X.; Sui, J.; Xiao, H.; Liu, M.; Wan, J.; Yu, W.W. Polyethylenimine modified magnetic graphene oxide nanocomposites for Cu2+ removal. RSC Adv. 2015, 5, 746–752. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, Q.; Sun, M.; Zhang, Y.; Jiang, H.; Lian, H. Fabrication and characterization of polyethyleneimine immobilized on chloropropyl- and silica-coated magnetic nanoparticles for Pb2+ removal from aqueous solution. Desalin. Water Treat. 2016, 57, 13701–13710. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Xing, H.; Zhao, J.; Li, X.; Huang, Y.; Liu, H. Synthesis of polyethylenimine-functionalized poly(glycidyl methacrylate) magnetic microspheres and their excellent Cr(VI) ion removal properties. Chem. Eng. J. 2013, 234, 338–345. [Google Scholar] [CrossRef]
- Sun, X.; Yang, L.; Li, Q.; Liu, Z.; Dong, T.; Liu, H. Polyethylenimine-functionalized poly(vinyl alcohol) magnetic microspheres as a novel adsorbent for rapid removal of Cr(VI) from aqueous solution. Chem. Eng. J. 2015, 262, 101–108. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, X.; Liu, Y.; Xu, B.; Pan, X. Highly stable and covalently functionalized magnetic nanoparticles by polyethyleneimine for Cr(VI) adsorption in aqueous solution. RSC Adv. 2015, 5, 1398–1405. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Chen, R.; Yu, J.; Zhang, H.; Liu, J.; Li, R.; Zhang, M.; Liu, P.; Wang, J. Efficient removal of U(vi) from simulated seawater with hyperbranched polyethylenimine (HPEI) covalently modified SiO2 coated magnetic microspheres. Inorg. Chem. Front. 2018, 5, 1321–1328. [Google Scholar] [CrossRef]
- Campelj, S.; Makovec, D.; Drofenik, M. Preparation and properties of water-based magnetic fluids. J. Phys. Condens. Matter 2008, 20, 204101. [Google Scholar] [CrossRef]
- Čakara, D.; Fras, L.; Bračič, M.; Kleinschek, K.S. Protonation behavior of cotton fabric with irreversibly adsorbed chitosan: A potentiometric titration study. Carbohydr. Polym. 2009, 78, 36–40. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Nie, H.-L.; Branford-White, C.; He, Z.-Y.; Zhu, L.-M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J. Colloid Interface Sci. 2009, 330, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Van Quy, D.; Hieu, N.M.; Tra, P.T.; Nam, N.H.; Hai, N.H.; Thai Son, N.; Nghia, P.T.; Van Anh, N.T.; Hong, T.T.; Luong, N.H. Synthesis of silica-coated magnetic nanoparticles and application in the detection of pathogenic viruses. J. Nanomater. 2013, 2013, 603940. [Google Scholar]
- Motevalizadeh, S.F.; Khoobi, M.; Sadighi, A.; Khalilvand-Sedagheh, M.; Pazhouhandeh, M.; Ramazani, A.; Faramarzi, M.A.; Shafiee, A. Lipase immobilization onto polyethylenimine coated magnetic nanoparticles assisted by divalent metal chelated ions. J. Mol. Catal. B Enzym. 2015, 120, 75–83. [Google Scholar] [CrossRef]
- Larkin, P.J. IR and Raman Spectroscopy—Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123869845. [Google Scholar]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 2013, 72, 90–98. [Google Scholar] [CrossRef]
- Finšgar, M.; Fassbender, S.; Hirth, S.; Milošev, I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater. Chem. Phys. 2009, 116, 198–206. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, M.; Zhao, J.; Chen, J.; Zeng, G.; Huang, H.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile preparation of polyethylenimine-tannins coated SiO2 hybrid materials for Cu2+ removal. Appl. Surf. Sci. 2018, 427, 535–544. [Google Scholar] [CrossRef]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76–77, 3–11. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lee, J.H.; Chung, J.W.; Kwak, S.Y. Hydrophilic and positively charged polyethylenimine-functionalized mesoporous magnetic clusters for highly efficient removal of Pb(II) and Cr(VI) from wastewater. J. Environ. Manag. 2018, 206, 740–748. [Google Scholar] [CrossRef]
- Tong, L.; Lu, E.; Pichaandi, J.; Cao, P.; Nitz, M.; Winnik, M.A. Quantification of Surface Ligands on NaYF4 Nanoparticles by Three Independent Analytical Techniques. Chem. Mater. 2015, 27, 4899–4910. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, X.; Mao, S.; Zhang, L.; Lu, J. Sub-micron-sized polyethylenimine-modified polystyrene/Fe3O4/chitosan magnetic composites for the efficient and recyclable adsorption of Cu(II) ions. Appl. Surf. Sci. 2017, 394, 378–385. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; John Wiley & Sons, Inc.: Toronto, ON, Canada, 1976; ISBN 0471039853. [Google Scholar]
- Perrine, T.D.; Landis, W.R. Analysis of polyethylenimine by spectrophotometry of its copper chelate. J. Polym. Sci. A1 1967, 5, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z. Enhanced selective removal of Cu(II) from aqueous solution by novel polyethylenimine-functionalized ion imprinted hydrogel: Behaviors and mechanisms. J. Hazard. Mater. 2015, 300, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiang, L.; Fan, J.; Cui, Z.; Wu, H. Synthesis of polyethylenimine-functionalized magnetic materials and a critical evaluation of the removal of copper in sediments. Environ. Earth Sci. 2018, 77, 441. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.L.; Shi, C.; Yu, H.Q.; Sheng, G.P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, Z.; Feng, L.; Luo, X.; Wu, P.; Cui, L.; Mao, X. Modified cellulose by polyethyleneimine and ethylenediamine with induced Cu(II) and Pb(II) adsorption potentialities. Carbohydr. Polym. 2018, 202, 470–478. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Zhang, X.; Lei, Z.; Chen, C.; Deng, Y.; Wang, C. Polyethylenimine and dithiocarbamate decorated melamine sponges for fast copper (II) ions removal from aqueous solution. Appl. Surf. Sci. 2018, 445, 471–477. [Google Scholar] [CrossRef]
- Yu, K.; Ho, J.; McCandlish, E.; Buckley, B.; Patel, R.; Li, Z.; Shapley, N.C. Copper ion adsorption by chitosan nanoparticles and alginate microparticles for water purification applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 31–41. [Google Scholar] [CrossRef]
- Taboada, E.; Cabrera, G.; Cardenas, G. Retention Capacity of Chitosan for Copper and Mercury Ions. J. Chil. Chem. Soc. 2003, 48, 7–12. [Google Scholar] [CrossRef]













| C | N | O | Si | Fe | |
|---|---|---|---|---|---|
| MNPs | 15.4 | 53.2 | 31.4 | ||
| MNPs@SiO2 | 7.4 | 65.4 | 24.4 | 3.3 | |
| MNPs@SiO2@GOPTS-bPEI | 29.5 | 9.4 | 43.4 | 16.0 | 1.9 |
| BET Surface Area * (m2·g−1) | BJH Adsorption Pore Volume (cm3·g−1) | |
|---|---|---|
| MNPs | 13.56 | / |
| MNPs@SiO2 | 25.21 | 0.16 |
| MNPs@SiO2@GOPTS-bPEI | 38.33 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plohl, O.; Finšgar, M.; Gyergyek, S.; Ajdnik, U.; Ban, I.; Zemljič, L.F. Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites. Nanomaterials 2019, 9, 209. https://doi.org/10.3390/nano9020209
Plohl O, Finšgar M, Gyergyek S, Ajdnik U, Ban I, Zemljič LF. Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites. Nanomaterials. 2019; 9(2):209. https://doi.org/10.3390/nano9020209
Chicago/Turabian StylePlohl, Olivija, Matjaž Finšgar, Sašo Gyergyek, Urban Ajdnik, Irena Ban, and Lidija Fras Zemljič. 2019. "Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites" Nanomaterials 9, no. 2: 209. https://doi.org/10.3390/nano9020209