Coordination Complex Transformation-Assisted Fabrication for Hollow Chestnut-Like Hierarchical ZnS with Enhanced Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Characterization
2.4. Photocatalytic Activity
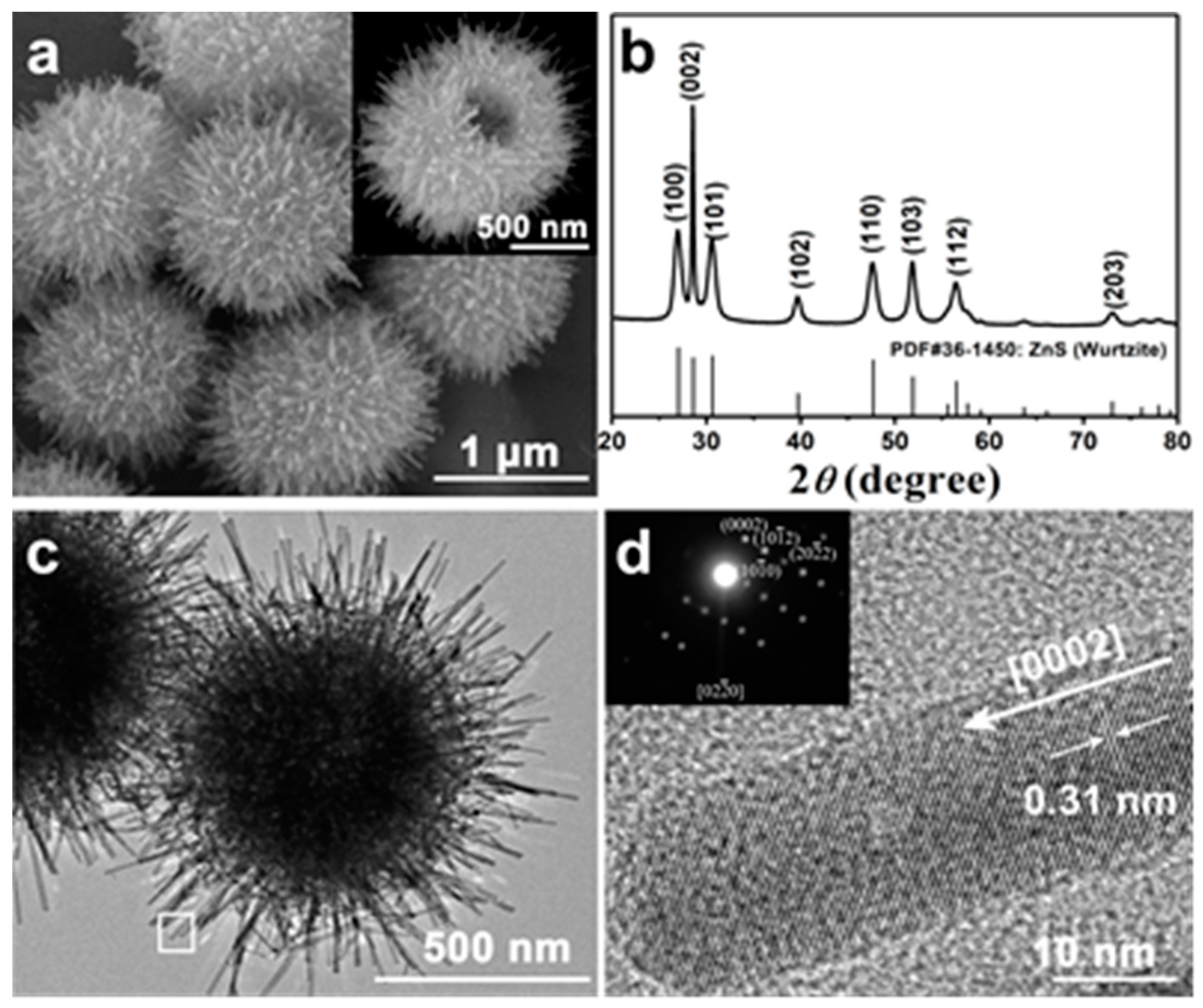
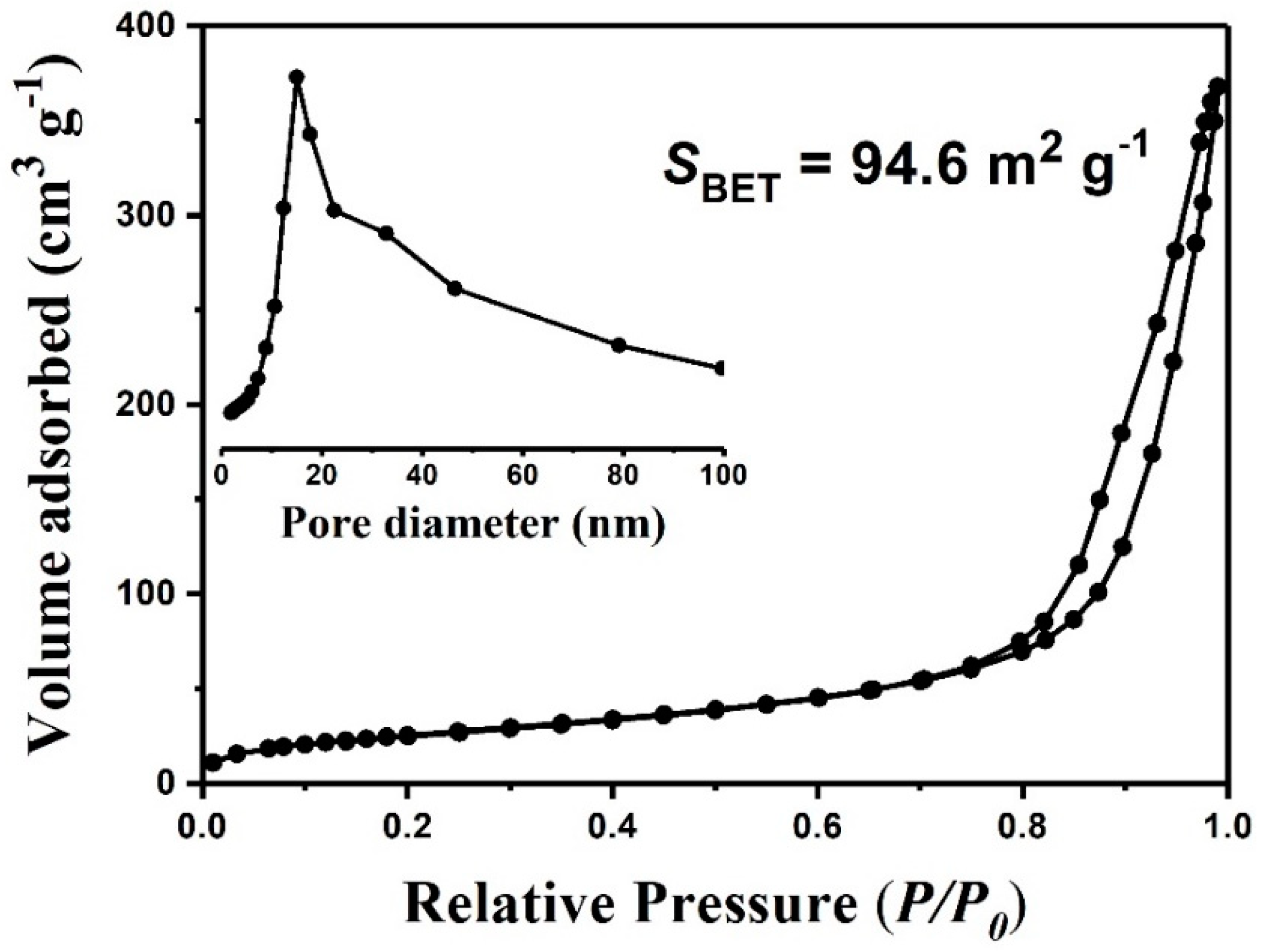
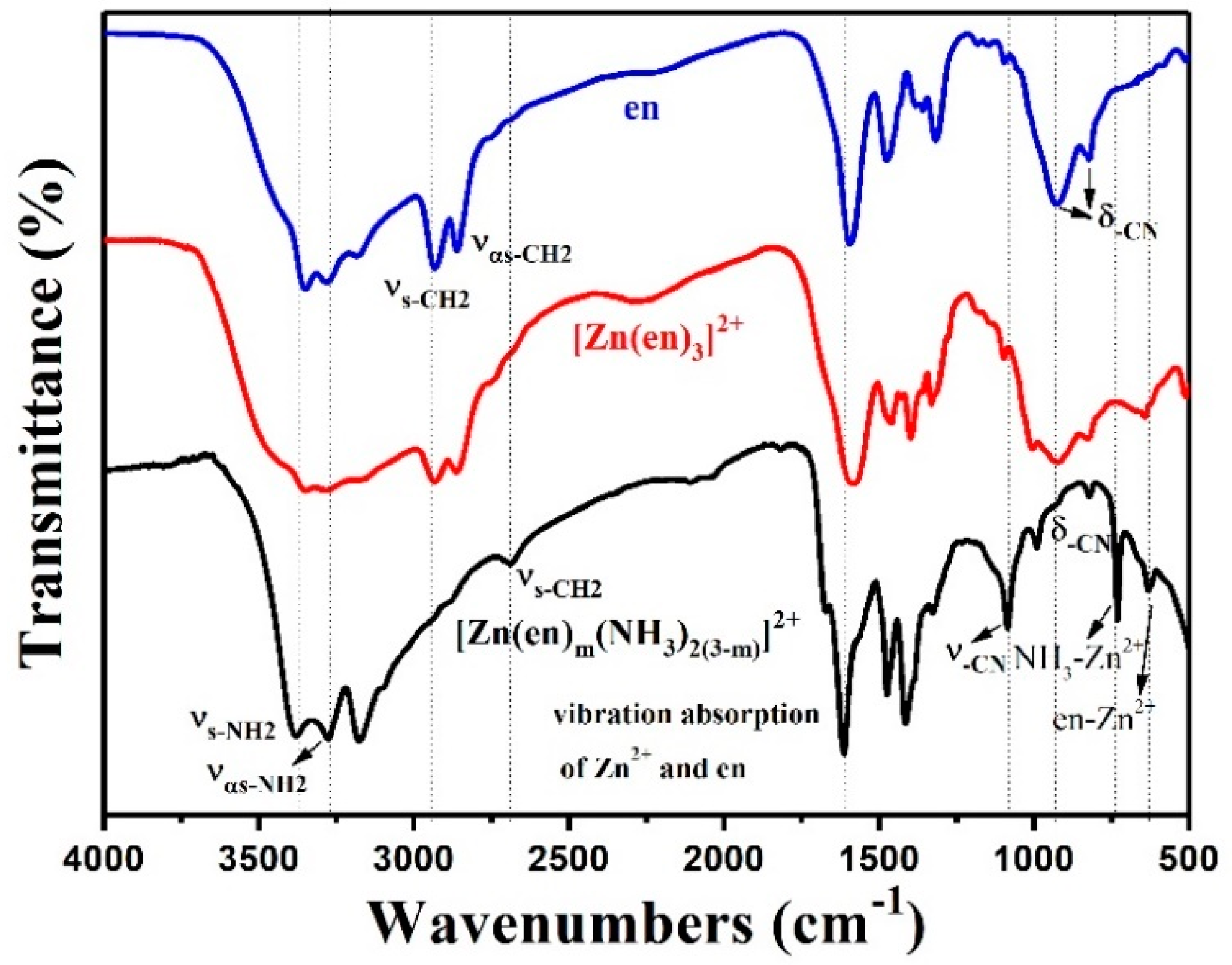
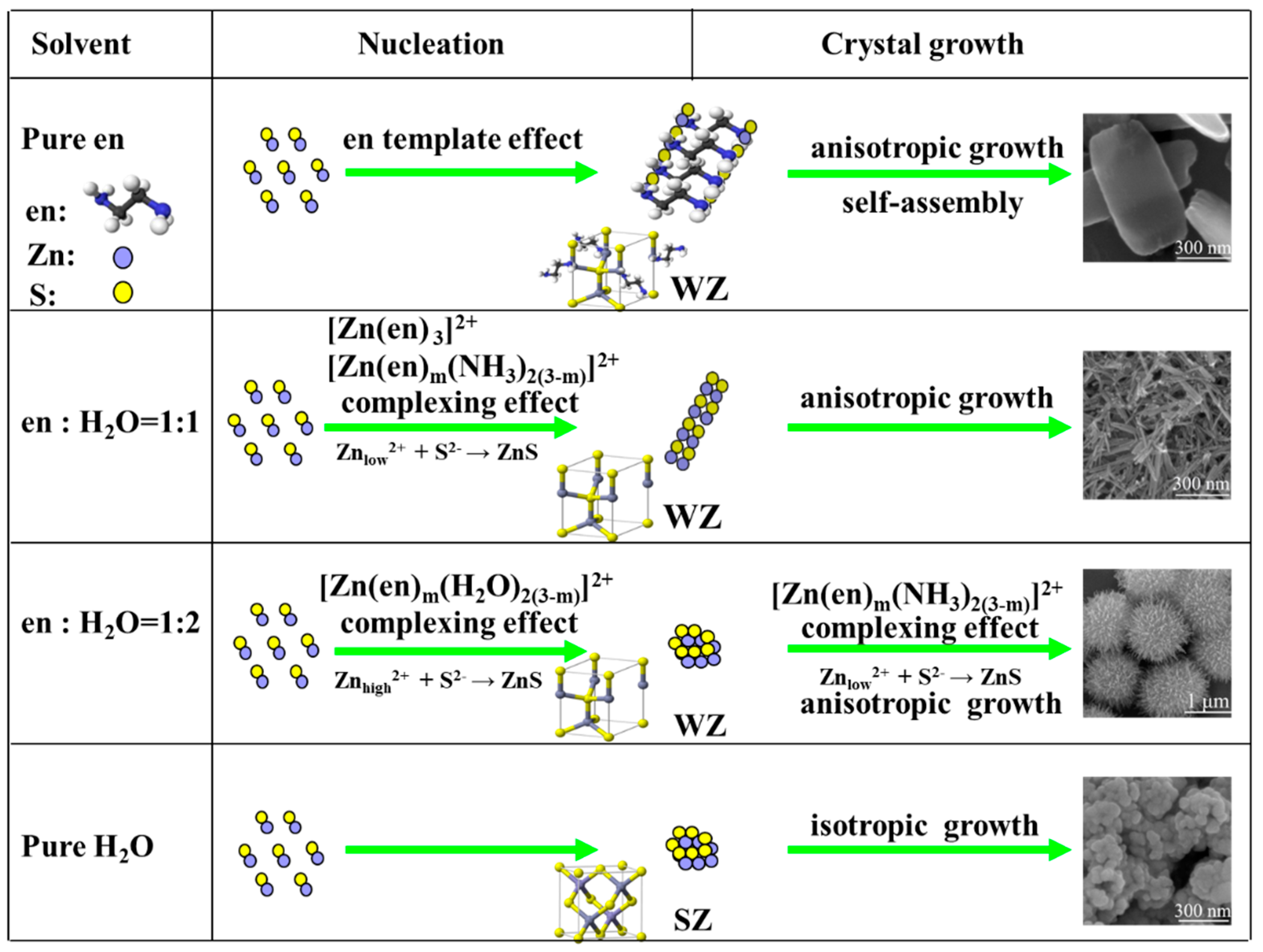
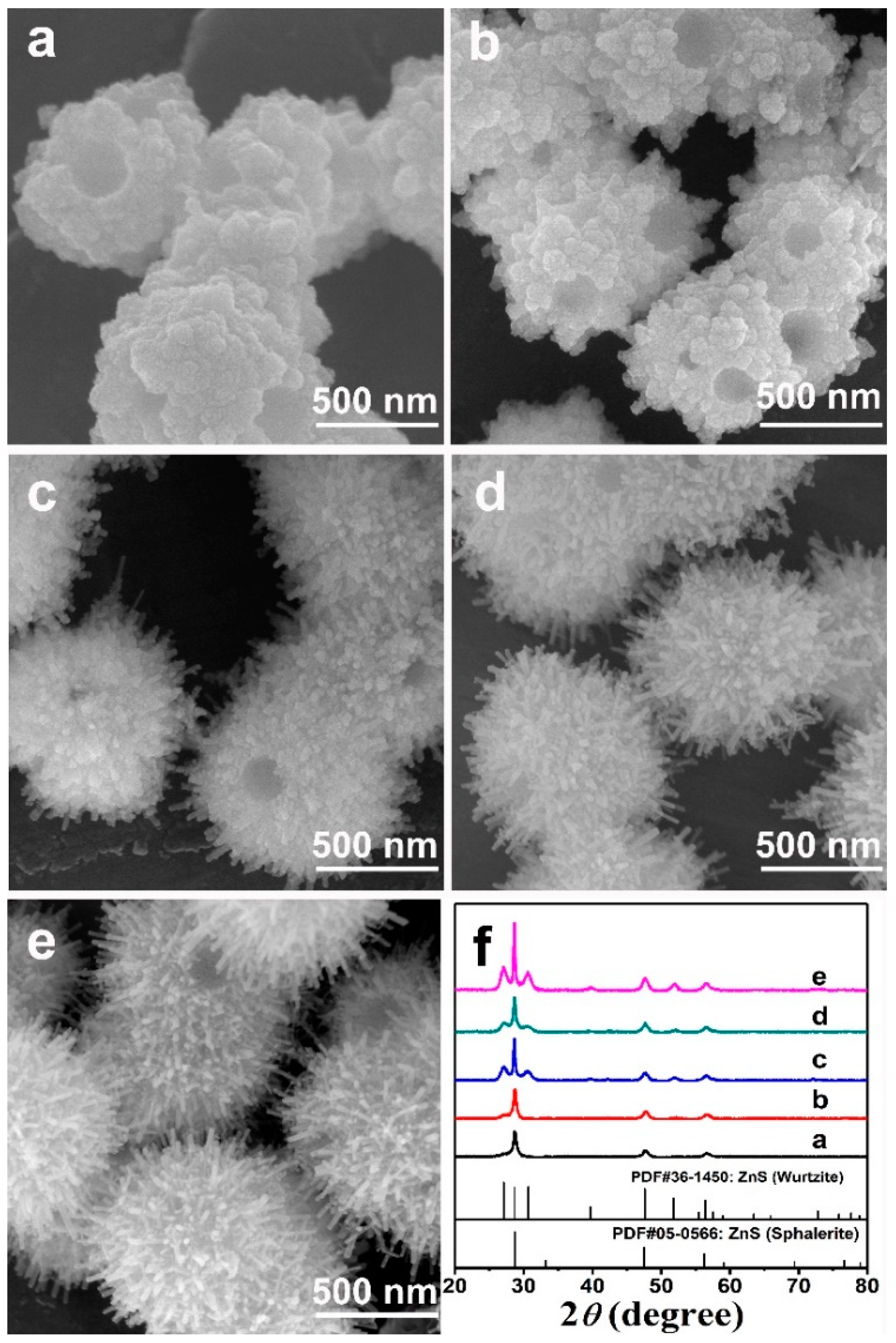
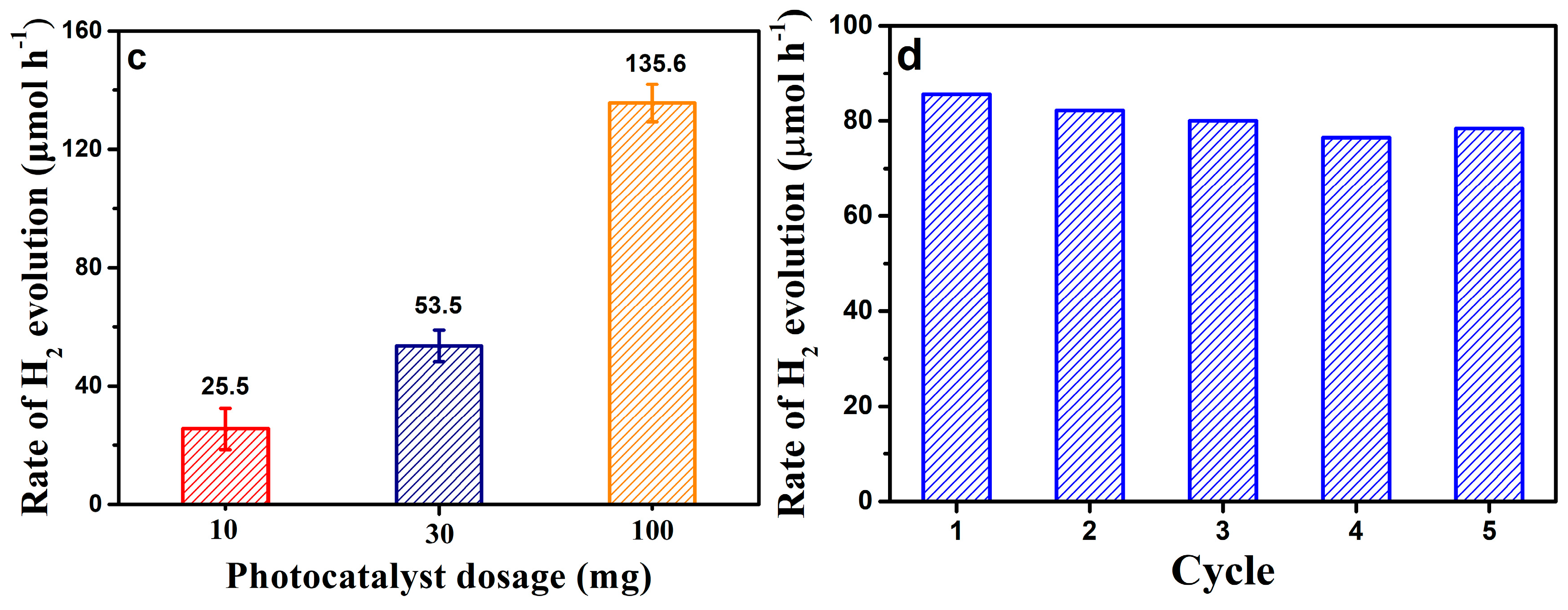
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mou, F.Z.; Guan, J.G.; Xiao, Z.D.; Sun, Z.G.; Shi, W.D.; Fan, X.A. Solvent-mediated Synthesis of Magnetic Fe2O3 Chestnut-like Amorphous-core/γ-phase-shell Hierarchical Nanostructures with Strong As(V) Removal Capability. J. Mater. Chem. 2011, 21, 5414–5421. [Google Scholar] [CrossRef]
- Liu, L.J.; Guan, J.G.; Shi, W.D.; Sun, Z.G.; Zhao, J.S. Facile Synthesis and Growth Mechanism of Flowerlike Ni−Fe Alloy Nanostructures. J. Phys. Chem. C 2010, 114, 13565–13570. [Google Scholar] [CrossRef]
- Pan, J.H.; Wang, X.Z.; Huang, Q. Large-scale Synthesis of Urchin-like mesoporous TiO2 Hollow Spheres by Targeted Etching and Their Photoelectrochemical Properties. Adv. Funct. Mater. 2014, 24, 95–104. [Google Scholar] [CrossRef]
- Liu, Y.F.; Huang, F.Q.; Xie, Y.; Cui, H.L.; Zhao, W.; Yang, C.Y.; Dai, N. Controllable Synthesis of Cu2In2ZnS5 Nano/Microcrystals and Hierarchical Films and Applications in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 10296–10301. [Google Scholar] [CrossRef]
- Ciszek, J.W.; Huang, L.; Wang, Y.; Mirkin, C.A. Kinetically Controlled, Shape-directed Assembly of Nanorods. Small 2008, 4, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.Y.; Shi, W.D.; Xu, L.L.; Mou, G.Y.; Xin, Q.L.; Guan, J.G. Hierarchical Nanostructures of Fluorinated and Naked Ta2O5 Single Crystalline Nanorods: Hydrothermal Preparation, Formation Mechanism and Photocatalytic Activity for H2 Production. Chem. Commun. 2012, 48, 7301–7303. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-Assembly of Metal Oxides into Three-Dimensional Nanostructures: Synthesis and Application in Catalysis. ACS Nano 2009, 3, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Duan, J.; Zhang, C. Sacrificial Template Synthesis and Photothermal Conversion Enhancements of Hierarchical and Hollow CuInS2 Microspheres. J. Phys. Chem. C 2013, 117, 9121–9128. [Google Scholar] [CrossRef]
- Zhu, F.F.; Yan, M.; Liu, Y.; Shen, H.; Lei, Y.; Shi, W.D. Hexagonal Prism-like Hierarchical Co9S8@Ni(OH)2 Core-shell Nanotubes on Carbon Fibers for High Performance Asymmetric Supercapacitors. J. Mater. Chem. A 2017, 5, 22782–22789. [Google Scholar] [CrossRef]
- Kanaras, A.G.; Sönnichsen, C.; Liu, H.; Alivisatos, A.P. Controlled Synthesis of Hyperbranched Inorganic Nanocrystals with Rich Three-dimensional Structures. Nano Lett. 2005, 5, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.E.; Li, M.; Mann, S.; Margolis, H.C. Influence of Surfactant Assembly on the Formation of Calcium Phosphate Materials-A Model for Dental Enamel Formation. J. Mater. Chem. 2005, 15, 3317–3325. [Google Scholar] [CrossRef]
- Sounart, T.L.; Liu, J.; Voigt, J.A.; Hsu, J.W.P.; Spoerke, E.D.; Tian, Z.; Jiang, Y.B. Sequential Nucleation and Growth of Complex Nanostructured Films. Adv. Funct. Mater. 2006, 16, 335–344. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Zhang, X. Controllable Synthesis of Stable Urchin-like Gold Nanoparticles Using Hydroquinone to Tune the Reactivity of Gold Chloride. J. Phys. Chem. C 2011, 115, 3630–3637. [Google Scholar] [CrossRef]
- Guan, J.G.; Liu, L.J.; Xu, L.L.; Sun, Z.G.; Zhang, Y. Nickel Flowerlike Nanostructures Composed of Nanoplates: One-pot Synthesis, Stepwise Growth Mechanism and Enhanced Ferromagnetic Properties. CrystEngComm 2011, 13, 2636–2643. [Google Scholar] [CrossRef]
- Tiwari, A.; Dhoble, S.J. Critical Analysis of Phase Evolution, Morphological Control, Growth Mechanism and Photophysical Applications of ZnS Nanostructures (Zero-Dimensional to Three-Dimensional): A Review. Cryst. Growth Des. 2017, 17, 381–407. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, A.P.; Yan, H.J.; Dong, Y.Y.; Tian, C.G.; Jiang, B.J.; Fu, H.G. Gelatin-assisted Synthesis of ZnS Hollow Nanospheres: The Microstructure Tuning, Formation Mechanism and Application for Pt-free Photocatalytic Hydrogen Production. CrystEngComm 2017, 19, 461–468. [Google Scholar] [CrossRef]
- Lin, H.M.; Parasuraman, P.S.; Ho, C.H. The Study of Near-band-edge Property in Oxygen-Incorporated ZnS for Acting as an Efficient Crystal Photocatalyst. ACS Omega 2018, 3, 6351–6359. [Google Scholar] [CrossRef]
- Bellotti, E.; Brennan, K.F.; Wang, R. Calculation of the Electron Initiated Impact Ionization Transition Rate in Cubic and Hexagonal Phase ZnS. J. Appl. Phys. 1997, 82, 2961–2964. [Google Scholar] [CrossRef]
- Acharya, S.A.; Maheshwari, N.; Tatikondewar, L.; Kshirsagar, A.; Klkarni, S.K. Ethylenediamine-Mediated Wurtzite Phase Formation in ZnS. Cryst. Growth Des. 2013, 13, 1369–1376. [Google Scholar] [CrossRef]
- Hong, Y.P.; Zhang, J.; Wang, X.; Wang, Y.J.; Zhang, L.; Yu, J.G.; Huang, F. Influence of Lattice Integrity and Phase Composition on the Photocatalytic Hydrogen Production Efficiency of ZnS Nanomaterials. Nanoscale 2012, 4, 2859–2862. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jang, D.-J. A Facile Growth Mechanism of Wurtzite ZnS Nanostructures Showing Intense Ultraviolet Luminescence. CrystEngComm 2014, 16, 6989–6995. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, H.R.; Wang, Q.B. Ultrathin Single Crystal ZnS Nanowires. Chem. Commun. 2010, 46, 8941–8943. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.T.; Yan, H.; Liu, Y. Controlled Colloidal Growth of Ultrathin Single-Crystal ZnS Nanowires with a Magic-Size Diameter. Angew. Chem. 2010, 122, 8877–8880. [Google Scholar] [CrossRef]
- Jiang, N.N.; Wu, R.; Li, J. Ethanol Amine-assisted Solvothermal Growth of Wurtzite-structured ZnS Thin Nanorods. J. Alloy. Compd. 2012, 536, 85–90. [Google Scholar] [CrossRef]
- Kole, A.K.; Tiwary, C.S.; Kumbhakar, P. Ethylenediamine Assisted Synthesis of Wurtzite Zinc Sulphide Nanosheets and Porous Zinc Oxide Nanostructures: Near White Light Photoluminescence Emission and Photocatalytic Activity under Visible Light Irradiation. CrystEngComm 2013, 15, 5515–5525. [Google Scholar] [CrossRef]
- Zhou, G.T.; Wang, X.C.; Yu, J.C. A Low-Temperature and Mild Solvothermal Route to the Synthesis of Wurtzite-Type ZnS with Single-Crystalline Nanoplate-like Morphology. Cryst. Growth Des. 2005, 5, 1761–1765. [Google Scholar] [CrossRef]
- Nasi, L.; Calestani, D.; Besagni, T.; Ferro, P.; Fabbri, F.; Licci, F.; Mosca, R. ZnS and ZnO Nanosheets from ZnS(en)0.5 Precursor: Nanoscale Structure and Photocatalytic Properties. J. Phys. Chem. C 2012, 116, 6960–6965. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R. An Efficient ZnS-UV Photocatalysts Generated in situ from ZnS(en)0.5 Hybrid During the H2 Production in Methanol-Water Solution. Int. J. Hydrog. Energy 2012, 37, 17002–17008. [Google Scholar] [CrossRef]
- Lee, J.; Ham, S.; Choi, D.; Jang, D.-J. Facile Fabrication of Porous ZnS Nanostructures with A Controlled Amount of S Vacancies for Enhanced Photocatalytic Performances. Nanoscale 2018, 10, 14254–14263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Li, X.; Zhu, B.Y.; Wang, J.S.; Lan, H.X.; Chen, X.B. Synthesis of Porous ZnS, ZnO and ZnS/ZnO Nanosheet and Their Photocatalytic Activity. RSC Adv. 2017, 7, 30956–30962. [Google Scholar] [CrossRef]
- Hao, X.Q.; Zhou, J.; Cui, Z.W.; Wang, Y.C.; Wang, Y.; Zou, Z. Zn-vacancy Mediated Electron-hole Separation in ZnS/g-C3N4 Heterojunction for Efficient Visible-light Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2018, 229, 41–51. [Google Scholar] [CrossRef]
- Kole, A.K.; Tiwary, C.S.; Kumbhakar, P. Morphology Controlled Synthesis of Wurtzite ZnS Nanostructures Through Simple Hydrothermal Method and Observation of White Light Emission from ZnO Obtained by Annealing the Synthesized ZnS Nanostructures. J. Mater. Chem. C 2014, 2, 4338–4346. [Google Scholar] [CrossRef]
- Feigl, A.A.; Barnard, A.S.; Russo, S.P. Size- and Shape-dependent Phase Transformations in Wurtzite ZnS Nanostructures. Phys. Chem. Chem. Phys. 2012, 14, 9871–9879. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.L.; Xi, B.J.; Wang, C.M.; Xu, D.H.; Feng, X.M.; Zhu, Z.C.; Qian, Y.T. Tunable Synthesis of Various Wurtzite ZnS Architectural Structures and Their Photocatalytic Properties. Adv. Funct. Mater. 2007, 17, 2728–2738. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Liu, X.J.; Willander, M. Hydrothermal Synthesis of Nanoclusters of ZnS Comprised on Nanowires. Nanomaterials 2013, 3, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, Z.P.; Wang, W.J.; Huang, Q.S.; Zhu, K.X.; Chen, X.L. Heterogeneous ZnS Hollow Urchin-like Hierarchical Nanostructures and Their Structure-enhanced Photocatalytic activity. Nanoscale 2011, 3, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.A.; Sinclair, H. On the Determination of Lattice Parameters by the Debye-scherrer Method. Proc. Phys. Soc. 1945, 57, 126–135. [Google Scholar] [CrossRef]
- Lu, F.; Cai, W.; Zhang, Y.G. ZnO Hierarchical Micro/Nanoarchitectures: Solvothermal Synthesis and Structurally Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2008, 18, 1047–1056. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Geng, F.X.; Cong, H.T.; Bai, J.; Cheng, H.M. A Simple Solution Route to Controlled Synthesis of ZnS Submicrospheres, Nanosheets and Nanorods. Nanotechnology 2006, 17, 4731–4735. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, P.R.; Cukrowski, I.; Marques, H.M. DFT-UX3LYP Studies on the Coordination Chemistry of Ni2+. Part 1: Six Coordinate [Ni(NH3)n(H2O)6−n]2+ Complex. J. Phys. Chem. A 2008, 112, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.X.; Guan, J.G.; Zhang, Q.J. In Situ Generated Gas Bubble-Directed Self-Assembly: Synthesis, and Peculiar Magnetic and Electrochemical Properties of Vertically Aligned Arrays of High-density Co3O4 Nanotubes. Adv. Funct. Mater. 2013, 23, 2406–2414. [Google Scholar] [CrossRef]
- Tong, G.X.; Guan, J.G.; Xiao, Z.D.; Mou, F.Z.; Wang, W.; Yan, G.Q. In Situ Generated H2 Bubble-Engaged Assembly: A One-Step Approach for Shape-Controlled Growth of Fe Nanostructures. Chem. Mater. 2008, 20, 3535–3539. [Google Scholar] [CrossRef]
- Takagi, R. Growth of Oxide Whiskers on Metals at High Temperature. J. Phys. Soc. Jpn. 1957, 12, 1212–1218. [Google Scholar] [CrossRef]
- Richter, T.M.M.; LeTonquesse, S.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Trigonal-Bipyramidal Coordination in First Ammoniates of ZnF2: ZnF2(NH3)3 and ZnF2(NH3)2. Inorg. Chem. 2016, 55, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, H.H.; Zhang, J.; Lin, Z.; Huang, F.; Yu, J.G. Enhanced Photocatalytic Hydrogen Production Activities of Au-Loaded ZnS Flowers. ACS Appl. Mater. Interfaces 2013, 5, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Muruganandham, M.; Amutha, R.; Repo, E.; Sillanpää, M.; Kusumoto, Y.; Abdulla-Al-Mamun, M.D. Controlled Mesoporous Self-assembly of ZnS Microsphere for Photocatalytic Degradation of Methyl Orange Dye. J. Photochem. Photobiol. A Chem. 2010, 216, 133–141. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Ao, Y.; Guan, B.; Xiang, Y.; Guan, J. Coordination Complex Transformation-Assisted Fabrication for Hollow Chestnut-Like Hierarchical ZnS with Enhanced Photocatalytic Hydrogen Evolution. Nanomaterials 2019, 9, 273. https://doi.org/10.3390/nano9020273
Xu L, Ao Y, Guan B, Xiang Y, Guan J. Coordination Complex Transformation-Assisted Fabrication for Hollow Chestnut-Like Hierarchical ZnS with Enhanced Photocatalytic Hydrogen Evolution. Nanomaterials. 2019; 9(2):273. https://doi.org/10.3390/nano9020273
Chicago/Turabian StyleXu, Leilei, Yuwei Ao, Bin Guan, Yun Xiang, and Jianguo Guan. 2019. "Coordination Complex Transformation-Assisted Fabrication for Hollow Chestnut-Like Hierarchical ZnS with Enhanced Photocatalytic Hydrogen Evolution" Nanomaterials 9, no. 2: 273. https://doi.org/10.3390/nano9020273




