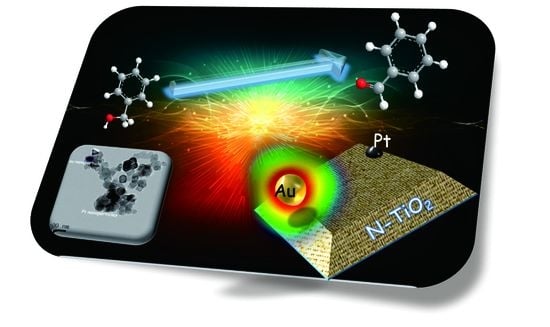
Coupling Plasmonic and Cocatalyst Nanoparticles on N–TiO2 for Visible-Light-Driven Catalytic Organic Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Sample Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Zhang, N.; Han, C.; Fu, X.; Xu, Y.-J. Function-Oriented Engineering of Metal-Based Nanohybrids for Photoredox Catalysis: Exerting Plasmonic Effect and Beyond. Chem 2018, 4, 1832–1861. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.; Jaatinen, E.; Xiao, Q.; Liu, H.; Jia, J.; Chen, C.; Zhao, J. Enhancing catalytic performance of palladium in gold and palladium alloy nanoparticles for organic synthesis reactions through visible light irradiation at ambient temperatures. J. Am. Chem. Soc. 2013, 135, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Jia, H.; Chang, S.; Ruan, Q.; Wang, P.; Chen, T.; Wang, J. (Gold core)/(titania shell) nanostructures for plasmon-enhanced photon harvesting and generation of reactive oxygen species. Energy Environ. Sci. 2014, 7, 3431–3438. [Google Scholar] [CrossRef]
- Zhang, N.; Han, C.; Xu, Y.-J.; Foley Iv, J.J.; Zhang, D.; Codrington, J.; Gray, S.K.; Sun, Y. Near-field dielectric scattering promotes optical absorption by platinum nanoparticles. Nat. Photonics 2016, 10, 473–482. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakanishi, K.; Hamada, R.; Hashimoto, K.; Kominami, H. Simultaneous and stoichiometric water oxidation and Cr(VI) reduction in aqueous suspensions of functionalized plasmonic photocatalyst Au/TiO2-Pt under irradiation of green light. ACS Catal. 2013, 3, 1886–1891. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Chang, X.; Gong, J. Effective Charge Carrier Utilization in Photocatalytic Conversions. Acc. Chem. Res. 2016, 49, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gu, T.; Ming, T.; Wang, J.; Wang, P.; Wang, J.; Yu, J.C. (Gold Core)@(Ceria Shell) Nanostructures for Plasmon-Enhanced Catalytic Reactions under Visible Light. ACS Nano 2014, 8, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photonics 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, T.D.; Gong, J. Mechanistic Understanding of the Plasmonic Enhancement for Solar Water Splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Fabbri, F.; Altomare, M.; Marelli, M.; Psaro, R.; Selli, E.; Salviati, G.; Dal Santo, V. The critical role of intragap states in the energy transfer from gold nanoparticles to TiO2. Phys. Chem. Chem. Phys. 2015, 17, 4864–4869. [Google Scholar] [PubMed]
- Wang, X.; Long, R.; Liu, D.; Yang, D.; Wang, C.; Xiong, Y. Enhanced full-spectrum water splitting by confining plasmonic Au nanoparticles in N-doped TiO2 bowl nanoarrays. Nano Energy 2016, 24, 87–93. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Wang, J.; Gan, Y.; Liu, L.; Ju, M. Microwave-assisted ionic liquid synthesis of Ti3+ self-doped TiO2 hollow nanocrystals with enhanced visible-light photoactivity. Appl. Catal. B Environ. 2016, 191, 94–105. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.; Ni, Y.; Su, M.; Xu, Z. A new sight on hydrogenation of F and N-F doped {001} facets dominated anatase TiO2 for efficient visible light photocatalyst. Appl. Catal. B Environ. 2012, 127, 28–35. [Google Scholar] [CrossRef]
- Usón, L.; Sebastian, V.; Mayoral, A.; Hueso, J.L.; Eguizabal, A.; Arruebo, M.; Santamaria, J. Spontaneous formation of Au–Pt alloyed nanoparticles using pure nano-counterparts as starters: A ligand and size dependent process. Nanoscale 2015, 7, 10152–10161. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dao, T.D.; Kodiyath, R.; Kang, Q.; Abe, H.; Nagao, T.; Ye, J. Plasmonic janus-composite photocatalyst comprising Au and C-TiO2 for enhanced aerobic oxidation over a broad visible-light range. Adv. Funct. Mater. 2014, 24, 7754–7762. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol-water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Mao, Y.; Xing, M.; Zhang, J. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xu, Y.J. Defect-mediated growth of noble-metal (Ag, Pt, and Pd) nanoparticles on TiO2 with oxygen vacancies for photocatalytic redox reactions under visible light. J. Phys. Chem. C 2013, 117, 17996–18005. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ohara, T.; Yasumoto, N.; Shiraishi, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Hot-Electron-Induced Highly Efficient O2 Activation by Pt Nanoparticles Supported on Ta2O5 Driven by Visible Light. J. Am. Chem. Soc. 2015, 137, 9324–9332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, W.; Sun, S.; Zhang, L.; Zheng, Y. Equilibrating the plasmonic and catalytic roles of metallic nanostructures in photocatalytic oxidation over Au-modified CeO2. ACS Catal. 2015, 5, 613–621. [Google Scholar] [CrossRef]
- Lu, D.; Ouyang, S.; Xu, H.; Li, D.; Zhang, X.; Li, Y.; Ye, J. Designing Au Surface-Modified Nanoporous-Single-Crystalline SrTiO3 to Optimize Diffusion of Surface Plasmon Resonance-Induce Photoelectron toward Enhanced Visible-Light Photoactivity. ACS Appl. Mater. Interfaces 2016, 8, 9506–9513. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Tsukamoto, D.; Sugano, Y.; Shiro, A.; Ichikawa, S.; Tanaka, S.; Hirai, T. Platinum nanoparticles supported on anatase titanium dioxide as highly active catalysts for aerobic oxidation under visible light irradiation. ACS Catal. 2012, 2, 1984–1992. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Varma, R.S. Selective Oxidation of Alcohols Using Photoactive VO@g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Sugano, Y.; Shiraishi, Y.; Tsukamoto, D.; Ichikawa, S.; Tanaka, S.; Hirai, T. Supported Au-Cu bimetallic alloy nanoparticles: An aerobic oxidation catalyst with regenerable activity by visible-light irradiation. Angew. Chem. Int. Ed. 2013, 52, 5295–5299. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Jia, C.; Zhang, L.; He, S.; Sang, Y.; Li, H.; Li, Y.; Xu, X.; Liu, H. Gold and gold-palladium alloy nanoparticles on heterostructured TiO2 nanobelts as plasmonic photocatalysts for benzyl alcohol oxidation. Nanoscale 2015, 7, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S.; Kitao, N.; Yoshida, N.; Sakura, T.; Azuma, M.; Ohue, H.; Sakata, Y. Selective photocatalytic oxidation of benzyl alcohol and its derivatives into corresponding aldehydes by molecular oxygen on titanium dioxide under visible light irradiation. J. Catal. 2009, 266, 279–285. [Google Scholar] [CrossRef]
- Higashimoto, S.; Suetsugu, N.; Azuma, M.; Ohue, H.; Sakata, Y. Efficient and selective oxidation of benzylic alcohol by O2 into corresponding aldehydes on a TiO2 photocatalyst under visible light irradiation: Effect of phenyl-ring substitution on the photocatalytic activity. J. Catal. 2010, 274, 76–83. [Google Scholar] [CrossRef]
- Kobayashi, H.; Higashimoto, S. DFT study on the reaction mechanisms behind the catalytic oxidation of benzyl alcohol into benzaldehyde by O2 over anatase TiO2 surfaces with hydroxyl groups: Role of visible-light irradiation. Appl. Catal. B Environ. 2015, 170–171, 135–143. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S.; Hashimoto, K.; Kominami, H. Preparation of Au/TiO2 with metal cocatalysts exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation under irradiation of visible light. ACS Catal. 2013, 3, 79–85. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Preparation of Au/CeO2 exhibiting strong surface plasmon resonance effective for selective or chemoselective oxidation of alcohols to aldehydes or ketones in aqueous suspensions under irradiation by green light. J. Am. Chem. Soc. 2012, 134, 14526–14533. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Liu, Z.; Bo, A.; Zavahir, S.; Sarina, S.; Bottle, S.; Riches, J.D.; Zhu, H. Catalytic transformation of aliphatic alcohols to corresponding esters in O2 under neutral conditions using visible-light irradiation. J. Am. Chem. Soc. 2015, 137, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, L.; Zhang, S.; Zhu, Y.; Zhao, J. Electron spin resonance spin-trapping detection of radical intermediates in N-doped TiO2-assisted photodegradation of 4-chlorophenol. J. Phys. Chem. B 2006, 110, 3061–3065. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, T.; Chang, X.; Cai, W.; Zhang, P.; Zhang, J.; Gong, J. Spatial separation of oxidation and reduction co-catalysts for efficient charge separation: Pt@TiO2@MnOx hollow spheres for photocatalytic reactions. Chem. Sci. 2016, 7, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Salmistraro, M.; Schwartzberg, A.; Bao, W.; Depero, L.E.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Triggering and monitoring plasmon-enhanced reactions by optical nanoantennas coupled to photocatalytic beads. Small 2013, 9, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Corma, A.; García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Christopher, P.; Xin, H.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef] [PubMed]




| Entry | Substrate | Product | Yield b (%) | Selectivity c (%) |
|---|---|---|---|---|
| 1 |  |  | 80.9 | 97.7 |
| 2 |  |  | 78.1 | 79.6 |
| 3 d |  |  | 87.9 | 97.9 |
| 4 |  |  | 63.8 | 76.9 |
| 5 e |  |  | 91.2 | 100 |
| 6 |  |  | 50.1 | 54.2 |
| 7 |  |  | 52.2 | 75.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, Y.; Hou, Q.; Ju, M.; Li, W. Coupling Plasmonic and Cocatalyst Nanoparticles on N–TiO2 for Visible-Light-Driven Catalytic Organic Synthesis. Nanomaterials 2019, 9, 391. https://doi.org/10.3390/nano9030391
Wang Y, Chen Y, Hou Q, Ju M, Li W. Coupling Plasmonic and Cocatalyst Nanoparticles on N–TiO2 for Visible-Light-Driven Catalytic Organic Synthesis. Nanomaterials. 2019; 9(3):391. https://doi.org/10.3390/nano9030391
Chicago/Turabian StyleWang, Yannan, Yu Chen, Qidong Hou, Meiting Ju, and Weizun Li. 2019. "Coupling Plasmonic and Cocatalyst Nanoparticles on N–TiO2 for Visible-Light-Driven Catalytic Organic Synthesis" Nanomaterials 9, no. 3: 391. https://doi.org/10.3390/nano9030391
APA StyleWang, Y., Chen, Y., Hou, Q., Ju, M., & Li, W. (2019). Coupling Plasmonic and Cocatalyst Nanoparticles on N–TiO2 for Visible-Light-Driven Catalytic Organic Synthesis. Nanomaterials, 9(3), 391. https://doi.org/10.3390/nano9030391