CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CuInTe2 Nanocrystals (2 mmol stearic acid) Synthesis
2.3. CuInTe2 Nanocrystals (4 mmol stearic acid) Synthesis
2.4. CuTe Nanocubes Synthesis
2.5. CuTe Nanorods Synthesis
2.6. Conversion of CuTe to CuInTe2
2.7. Materials Characterization
2.8. Device Fabrication
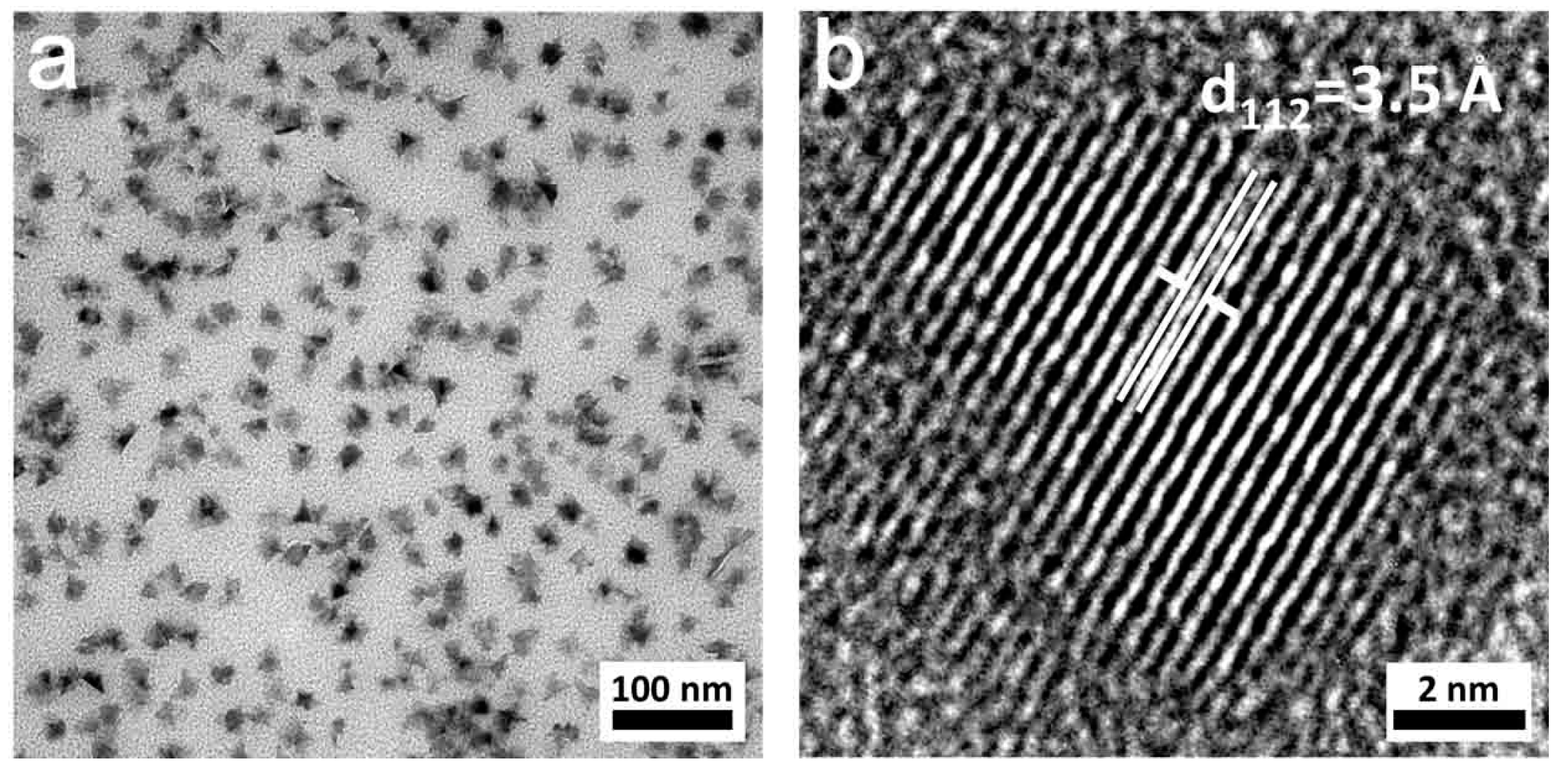
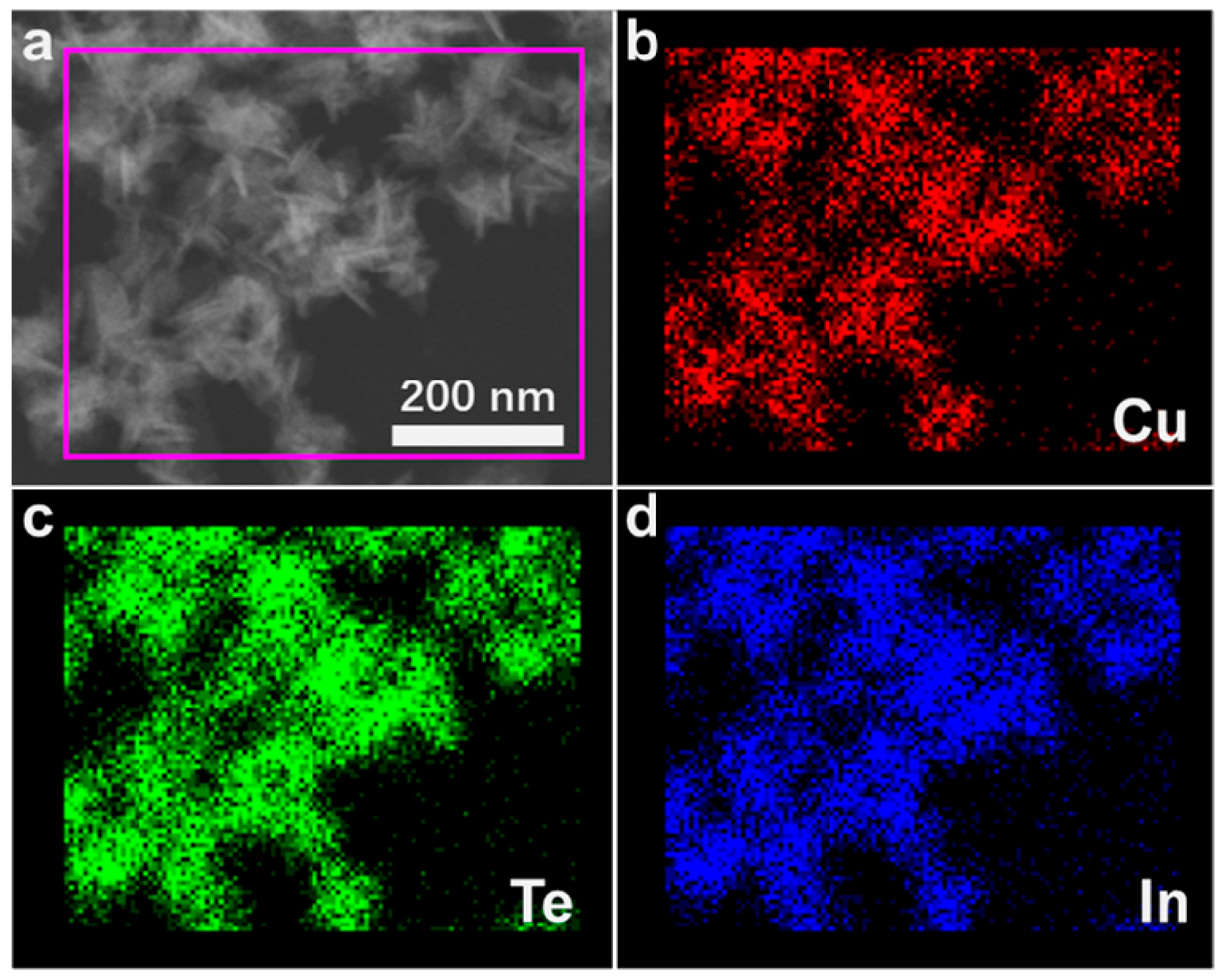
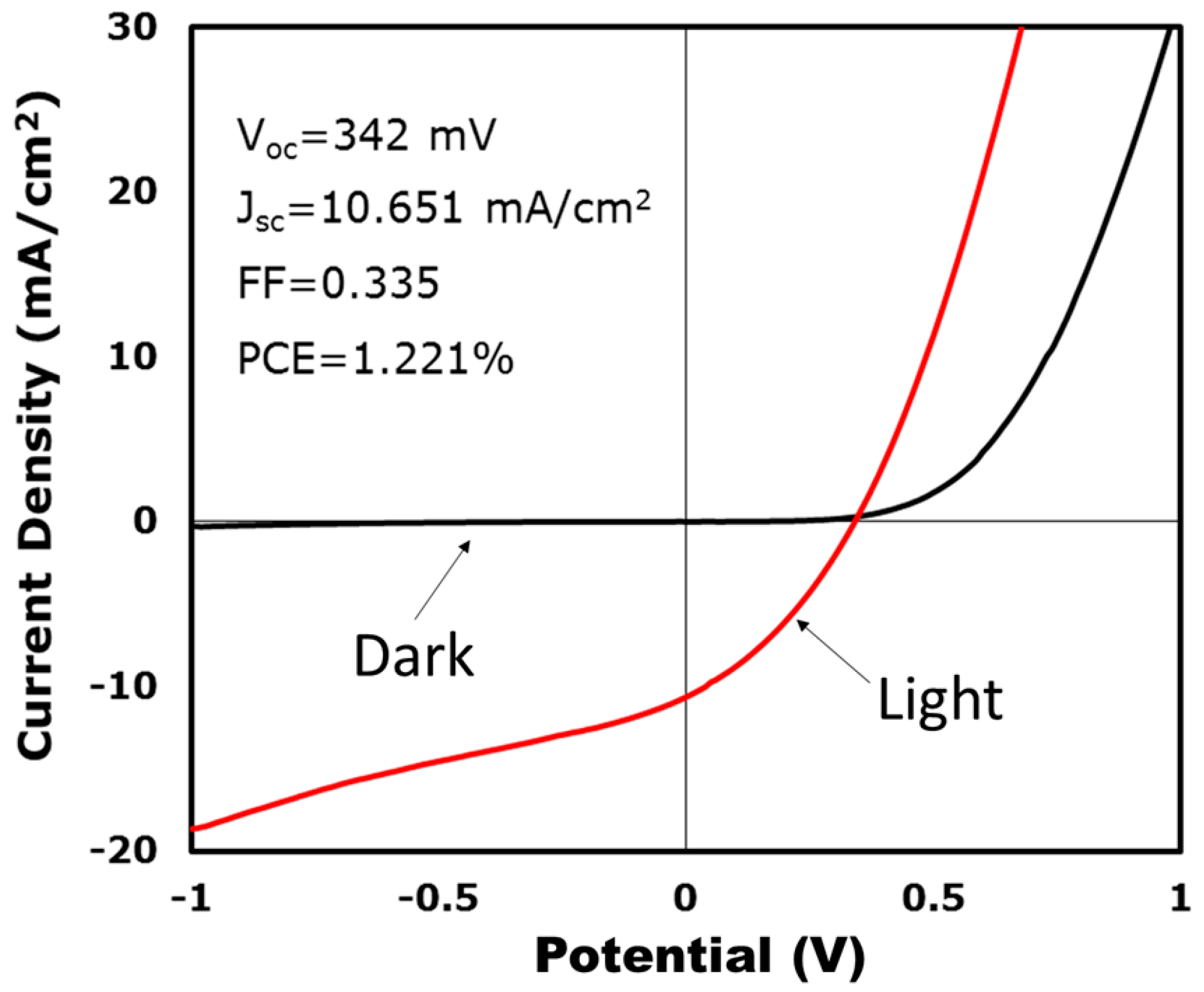
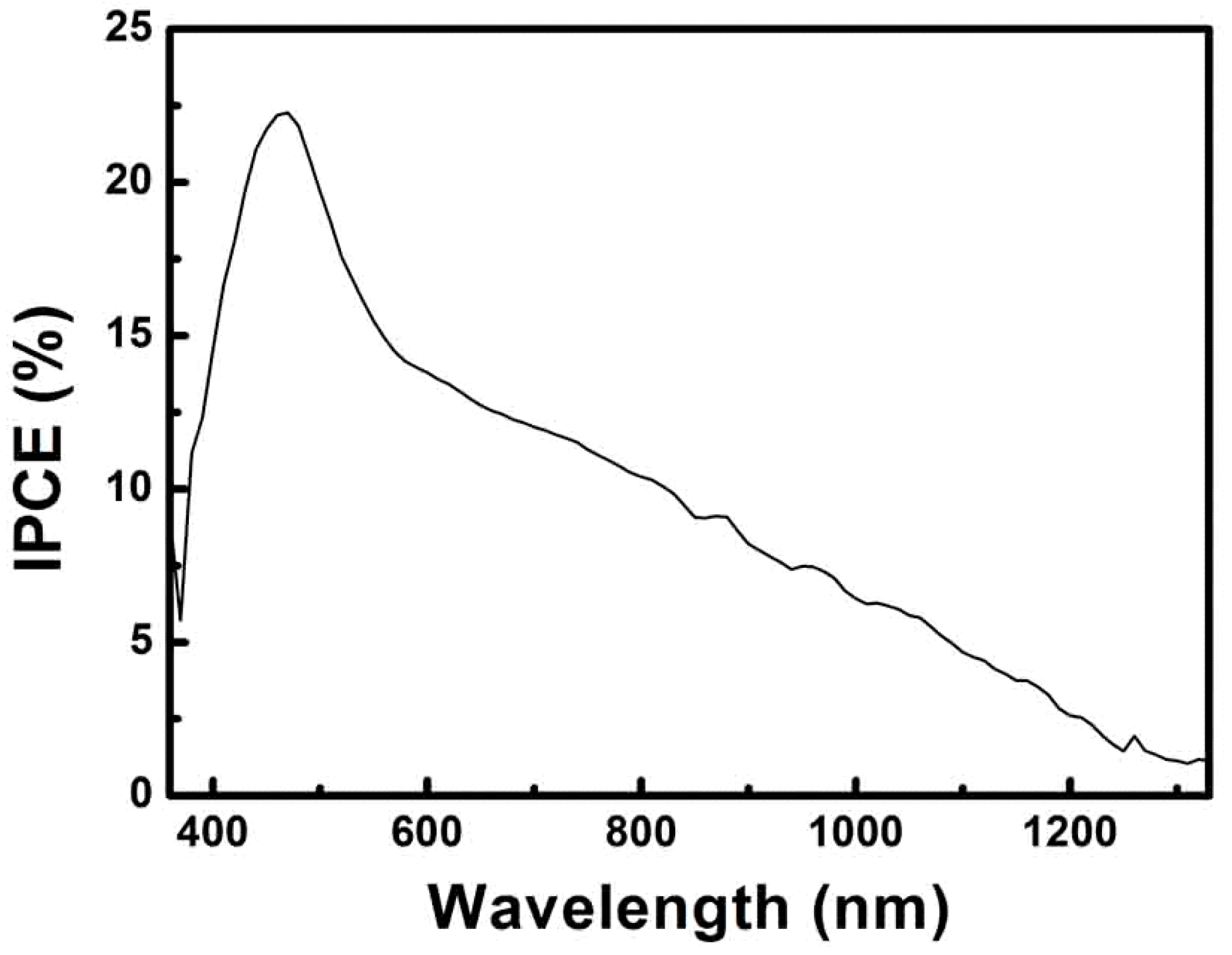
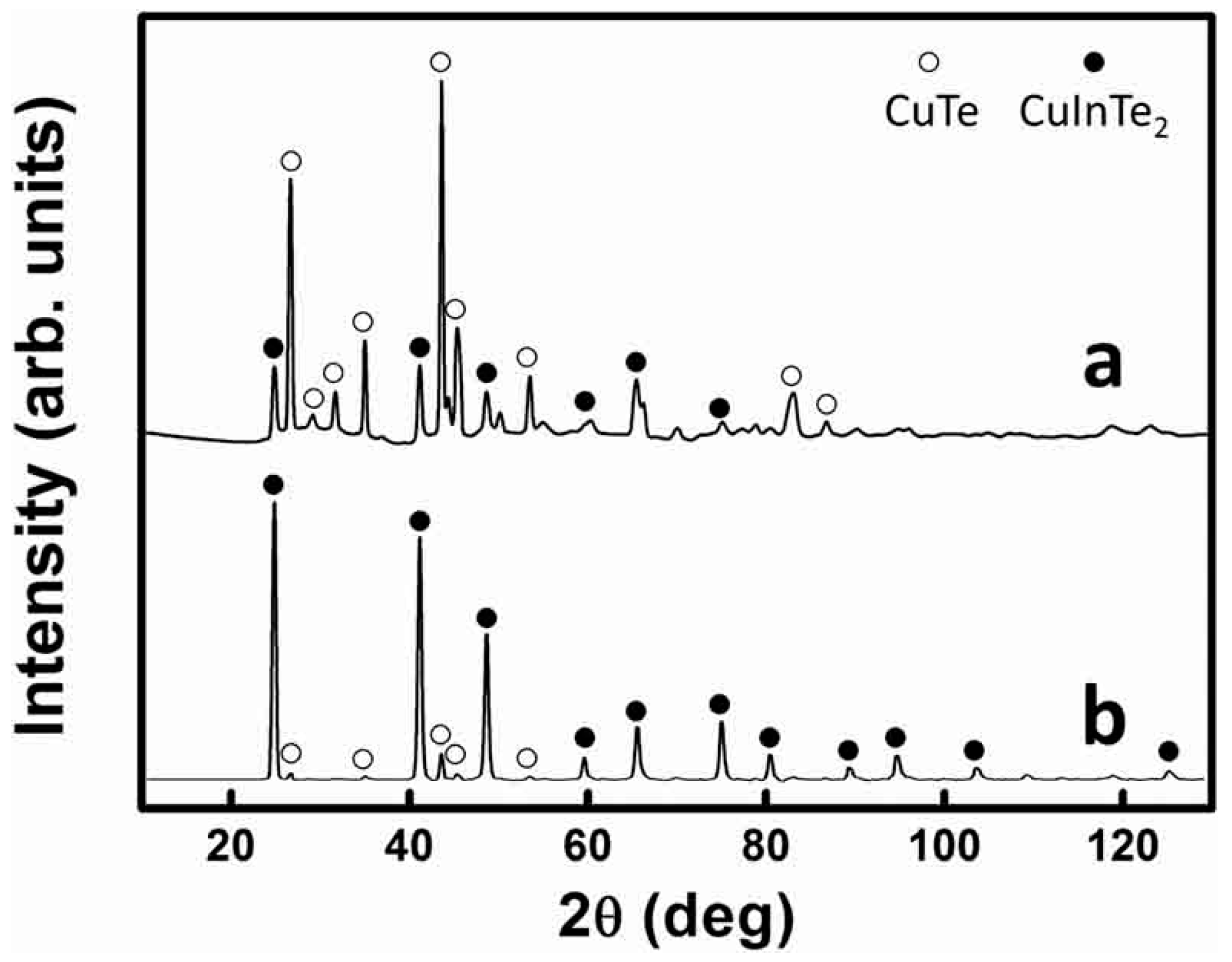
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stanbery, B. Copper Indium Selenides and related materials for photovoltaic devices. Crit. Rev. Solid State 2002, 27, 73–117. [Google Scholar] [CrossRef]
- Cha, J.H.; Noh, S.J.; Jung, D.Y. Synthesis and nanostructures of metal selenide precursors for Cu(In,Ga)Se2 thin-film solar cells. ChemSusChem 2015, 8, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lai, X.Y.; Halpert, J.E.; Yu, Y.; Wang, D. Formation of efficient dye-sensitized solar cells by introducing an interfacial layer of hierarchically ordered macro-mesoporous TiO2 film. Sci. China Chem. 2011, 54, 930–935. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Guo, Z.; Su, G.; Gao, R.; Wang, W.; Dong, B.; Cao, L. Rapid synthesis of CuInTe2 ultrathin nanoplates with enhanced photoelectrochemical properties. Chem. Commun. 2017, 53, 5878–5881. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Hariskos, D.; Lotter, E.; Paetel, S.; Wuerz, R.; Menner, R.; Wischmann, W.; Powalla, M. New world record efficiency for Cu(In,Ga)Se2 thin-film solar cells beyond 20%. Prog. Photovolt. Res. Appl. 2011, 19, 894–897. [Google Scholar] [CrossRef]
- Liu, R.; Xi, L.; Liu, H.; Shi, X.; Zhang, W.; Chen, L. Ternary compound CuInTe2: A promising thermoelectric material with diamond-like structure. Chem. Commun. 2012, 48, 3818–3820. [Google Scholar] [CrossRef] [PubMed]
- Rincón, C.; Wasim, S.M.; Marí́N, G.; Pérez, G.S.; Bacquet, G. Temperature dependence of the photoluminescence spectra of single crystals of CuInTe2. J. Appl. Phys. 1997, 82, 4500–4503. [Google Scholar] [CrossRef]
- Jia, G.; Du, J. Foreign metal ions to control the morphology of solution−liquid−solid reaction. Cryst. Growth Des. 2018, 18, 7489–7495. [Google Scholar] [CrossRef]
- Jia, G.; Du, J. Solution–liquid–solid growth of CuInTe2 and CuInSexTe2−x semiconductor nanowires. Inorg. Chem. 2018, 57, 14961–14966. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Du, J. Catalyst-assisted solution–liquid–solid synthesis of CdS/CuInSe2 and CuInTe2/CuInSe2 nanorod heterostructures. Inorg. Chem. 2019, 58, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Mise, T.; Nakada, T. Microstructural and optical properties of CuIn3Te5 thin films for solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1132–1136. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M.; Kim, S.; Heo, J.H.; Noh, J.H.; Im, S.H.; Seok, S.I.; Kim, S.W. Fabrication of CuInTe2 and CuInTe(2−x)Se(x) ternary gradient quantum dots and their application to solar cells. ACS Nano 2013, 7, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Lakhe, M.; Chaure, N.B. Characterization of electrochemically deposited CuInTe2 thin films for solar cell applications. Sol. Energy Mater. Sol. Cells 2014, 123, 122–129. [Google Scholar] [CrossRef]
- Shen, H.; Duong, T.; Peng, J.; Jacobs, D.; Wu, N.; Gong, J.; Wu, Y.; Karuturi, S.K.; Xiao, F.; Weber, K. Mechanically-stacked perovskite/CIGS Tandem solar cells with efficiency of 23.9% and reduced oxygen sensitivity. Energy Environ. Sci. 2018, 11, 394–406. [Google Scholar] [CrossRef]
- Du, J.; Jian, Q.; Wang, D.; Tang, Z. Facile synthesis of Au@TiO2 core-shell hollow spheres for dye-sensitized solar cells with remarkably improved efficiency. Energy Environ. Sci. 2012, 5, 6914–6918. [Google Scholar] [CrossRef]
- Roy, S.; Guha, P.; Chaudhuri, S.; Pal, A.K. CuInTe2 thin films synthesized by graphite box annealing of In/Cu/Te stacked elemental layers. Vacuum 2002, 65, 27–37. [Google Scholar] [CrossRef]
- Kazmerski, L.L.; Shieh, C.C. Photoconductivity effects in CuInS2, CuInSe2 and CuInTe2 thin films. Thin Solid Films 1977, 41, 35–41. [Google Scholar] [CrossRef]
- Mise, T.; Nakada, T. Low temperature growth and properties of Cu-In-Te based thin films for narrow bandgap solar cells. Thin Solid Films 2010, 518, 5604–5609. [Google Scholar] [CrossRef]
- Bodnar’, I.V.; Gurin, V.S.; Soloveĭ, N.P.; Molochko, A.P. Formation and optical properties of CuInTe2 nanoparticles in silicate matrices. Semiconductors 2007, 41, 939–945. [Google Scholar] [CrossRef]
- Panthani, M.G.; Akhavan, V.; Goodfellow, B.; Schmidtke, J.P.; Dunn, L.; Dodabalapur, A.; Barbara, P.F.; Korgel, B.A. Synthesis of CuInS, CuInSe, and Cu(InGa)Se (CIGS) nanocrystal “inks” for printable photovoltaics. J. Am. Chem. Soc. 2008, 130, 16700–16777. [Google Scholar] [CrossRef] [PubMed]
- Grisaru, H.; Palchik, O.; Gedanken, A.; Palchik, V.; Slifkin, M.A.; Weiss, A.M. Microwave-Assisted polyol synthesis of CuInTe2 and CuInSe2 nanoparticles. Inorg. Chem. 2003, 42, 7148–7155. [Google Scholar] [CrossRef] [PubMed]
- Yarema, O.; Yarema, M.; Lin, W.M.; Wood, V. Cu-In-Te and Ag-In-Te colloidal nanocrystals with tunable composition and size. Chem. Commun. 2016, 52, 10878–10881. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Dong, H.K.; Son, Y.S.; Kim, H.; Bae, J.Y.; Han, Y.S. Solvothermal synthesis and characterization of a CuInTe2 absorber for thin-film photovoltaics. Mater. Res. Bull. 2012, 47, 4054–4058. [Google Scholar] [CrossRef]
- Steinhagen, C.; Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Harris, J.T.; Holmberg, V.C.; Korgel, B.A. Solution-liquid-solid synthesis of CuInSe2 nanowires and their implementation in photovoltaic devices. ACS Appl. Mater. Interfaces 2011, 3, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Nadenau, V.; Walter, T.; Schock, H.W. Growth of CuInTe2 polycrystalline thin films. J. Cryst. Growth 1995, 146, 251–255. [Google Scholar] [CrossRef]
- Ananthan, M.R.; Mohanty, B.C.; Kasiviswanathan, S. Micro-Raman spectroscopy studies of bulk and thin films of CuInTe2. Semicond. Sci. Technol. 2009, 24, 230–278. [Google Scholar] [CrossRef]
- Stanbery, B.J.; Kincal, S.; Kim, W.K.; Chang, C.H.; Ahrenkiel, S.P.; Lippold, G.; Neumann, H.; Anderson, T.J.; Crisalle, O.D. Epitaxial growth and characterization of CuInSe crystallographic polytypes. J. Appl. Phys. 2002, 91, 3598–3604. [Google Scholar] [CrossRef]
- Shirakata, S.; Murakami, T.; Kariya, T.; Isomura, S. Preparation of CuInSe2 thin films by chemical spray pyrolysis. Jpn. J. Appl. Phys. 1996, 35, 191–199. [Google Scholar] [CrossRef]
- Neumann, H. Lattice vibrations in AIBIIICVI2 chalcopyrite compounds. Helvetica Phys. Acta 1985, 58, 337–346. [Google Scholar]
- Neumann, H.; Nowak, E.; Schumann, B.; Tempel, A.; Kuhn, G. Epitaxial layers of CuInTe2 on GaAs. Cryst. Res. Technol. 1980, 15, 61–69. [Google Scholar] [CrossRef]
- Palatnik, L.S.; Rogacheva, E.I. Phase diagrams and structure of some semiconductor A2ICVI–B2IIICVI alloys. Sov. Phys. Dokl. 1967, 12, 503–506. [Google Scholar]
- Klenk, R.; Walter, T.; Schock, H.W.; Cahen, D. A model for the successful growth of polycrystalline films of CuInSe2 by multisource physical vacuum evaporation. Adv. Mater. 1993, 5, 114–119. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.H.; Zunger, A. A phenomenological model for systematization and prediction of doping limits in II–VI and I–III–VI2 compounds. J. Appl. Phys. 1998, 83, 3192–3196. [Google Scholar] [CrossRef]
- Nakamura, K.; Gotoh, M.; Fujihara, T.; Toyama, T.; Okamoto, H. Influence of CdS window layer on 2-μm thick CdS/CdTe thin film solar cells. Sol. Energy Mater. Sol. Cell 2003, 75, 185–192. [Google Scholar] [CrossRef]
- Neuschitzer, M.; Sanchez, Y.; López-Marino, S.; Xie, H.; Fairbrother, A.; Placidi, M.; Saucedo, E. Optimization of CdS buffer layer for high-performance Cu2ZnSnSe4 solar cells and the effects of light soaking: Elimination of crossover and red kink. Prog. Photovolt. Res. Appl. 2015, 23, 1660–1667. [Google Scholar] [CrossRef]
- Ericson, T.; Scragg, J.J.; Hultqvist, A.; Watjen, J.T.; Szaniawski, P.; Torndahl, T.; Platzer-Bjorkman, C. Zn(O, S) Buffer Layers and Thickness Variations of CdS Buffer for Cu2ZnSnS4 Solar Cells. IEEE J. Photovolt. 2014, 4, 465–469. [Google Scholar] [CrossRef]
- Wilkins, R.G.; Burkin, A.R. Complexes between metal salts and long-chain aliphatic amines. Part II. The complexes of cuprous halides with long-chain aliphatic amines. J. Chem. Soc. 1950, 27, 127–132. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, G.; Liu, B.; Wang, K.; Wang, C.; Yang, P.; Liu, J.; Zhang, W.; Li, R.; Zhang, S.; Du, J. CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics. Nanomaterials 2019, 9, 409. https://doi.org/10.3390/nano9030409
Jia G, Liu B, Wang K, Wang C, Yang P, Liu J, Zhang W, Li R, Zhang S, Du J. CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics. Nanomaterials. 2019; 9(3):409. https://doi.org/10.3390/nano9030409
Chicago/Turabian StyleJia, Guanwei, Baokun Liu, Kun Wang, Chengduo Wang, Peixu Yang, Jinhui Liu, Weidong Zhang, Rongbin Li, Shaojun Zhang, and Jiang Du. 2019. "CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics" Nanomaterials 9, no. 3: 409. https://doi.org/10.3390/nano9030409
APA StyleJia, G., Liu, B., Wang, K., Wang, C., Yang, P., Liu, J., Zhang, W., Li, R., Zhang, S., & Du, J. (2019). CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics. Nanomaterials, 9(3), 409. https://doi.org/10.3390/nano9030409




