Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate
Abstract
:1. Introduction
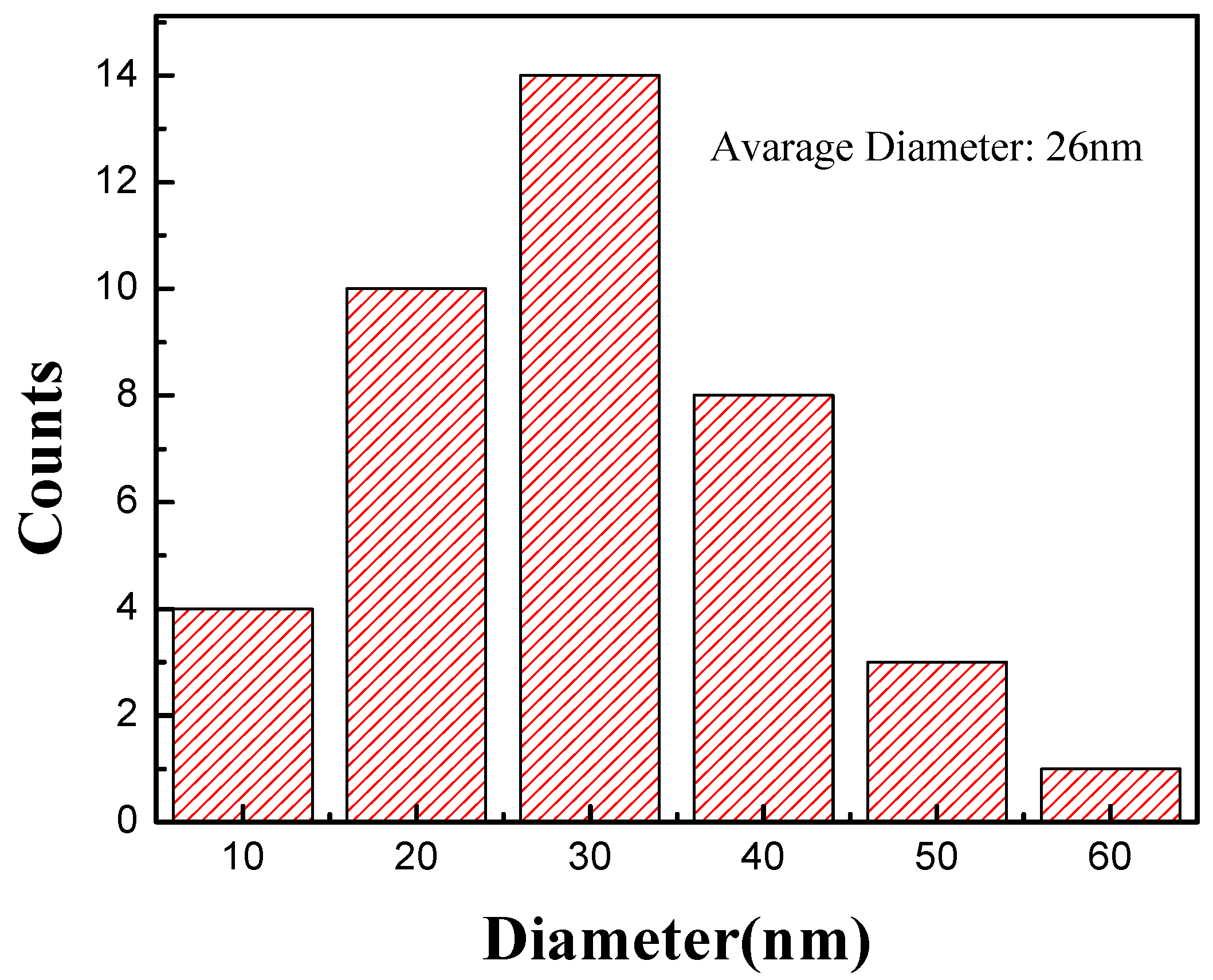
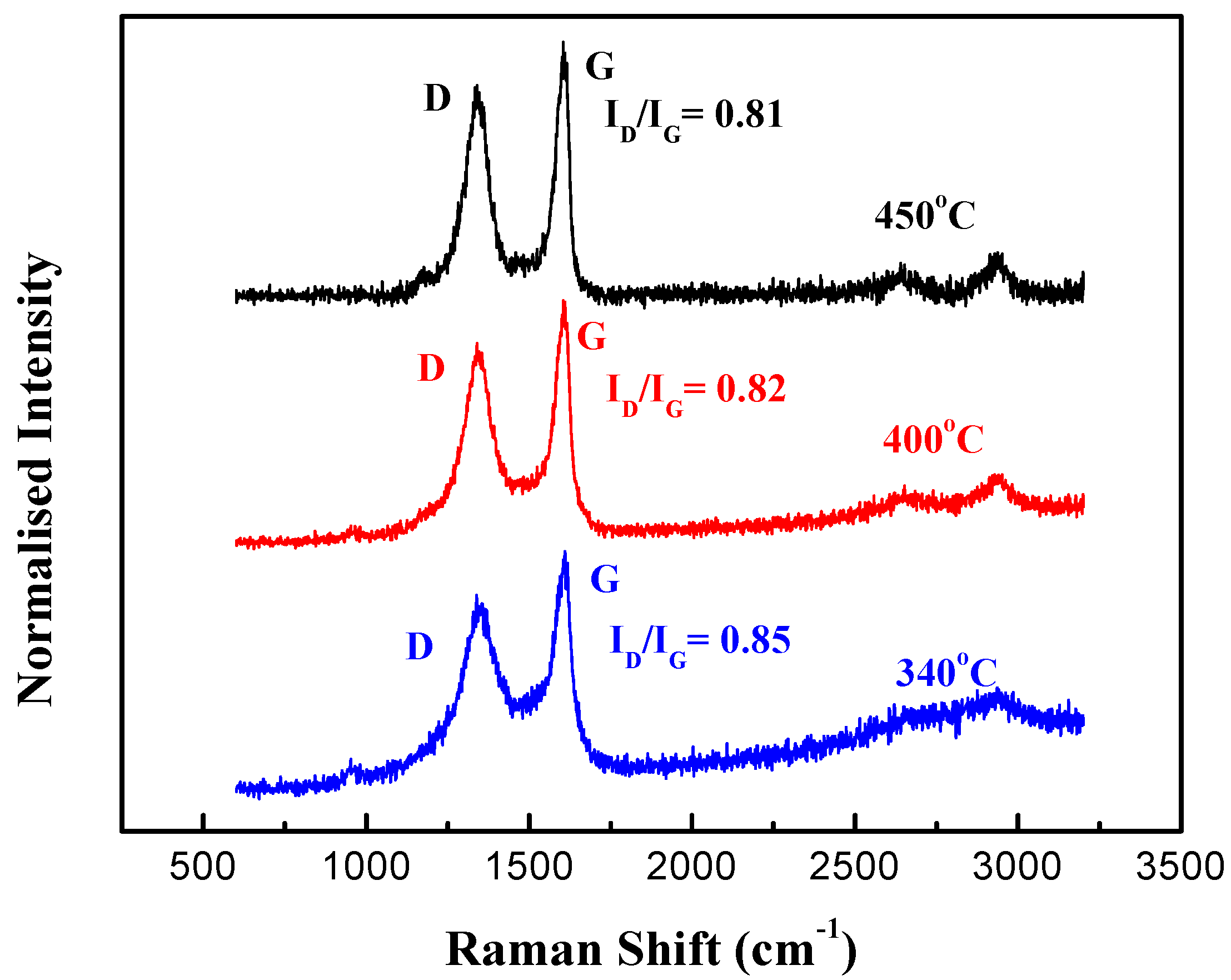
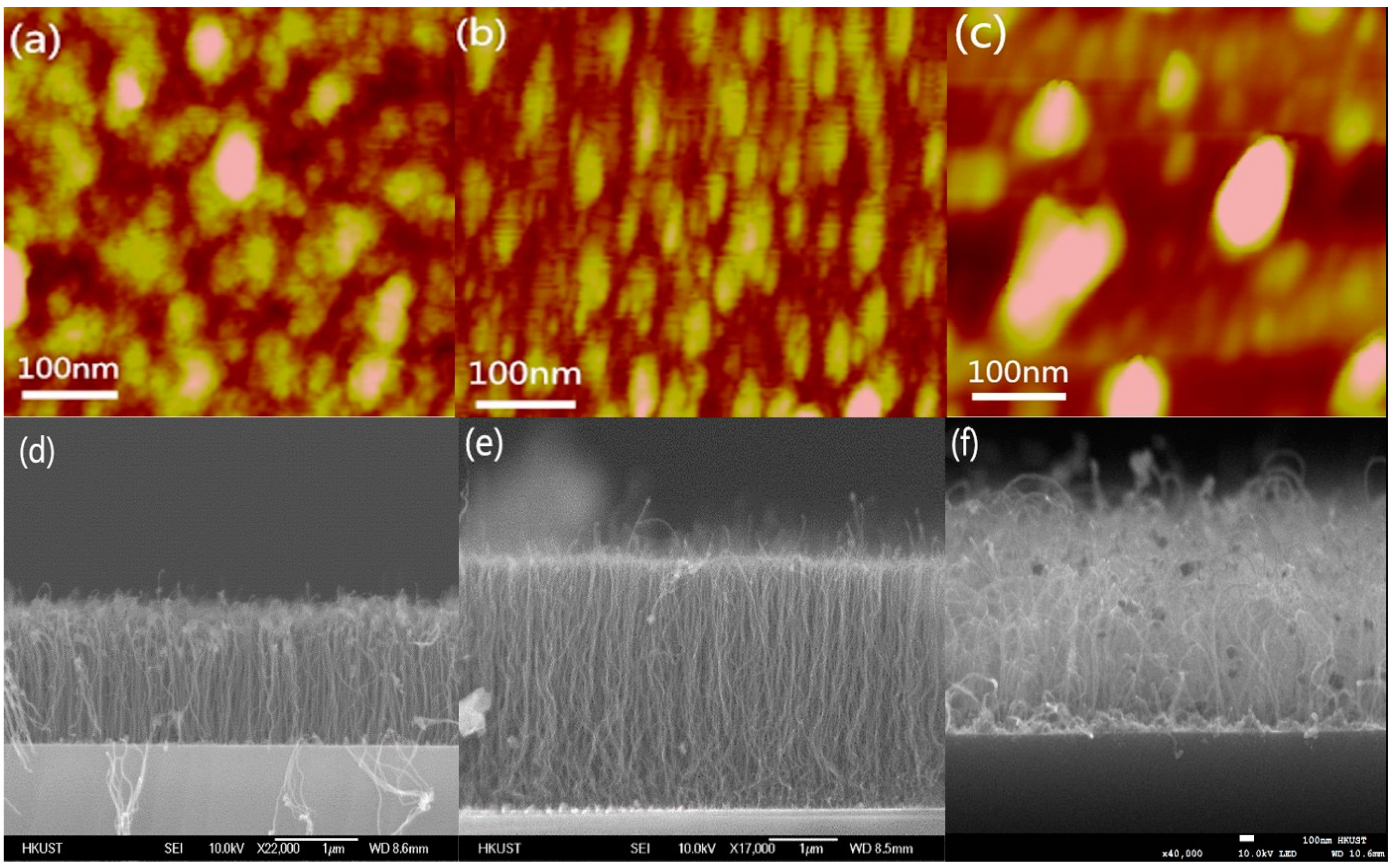
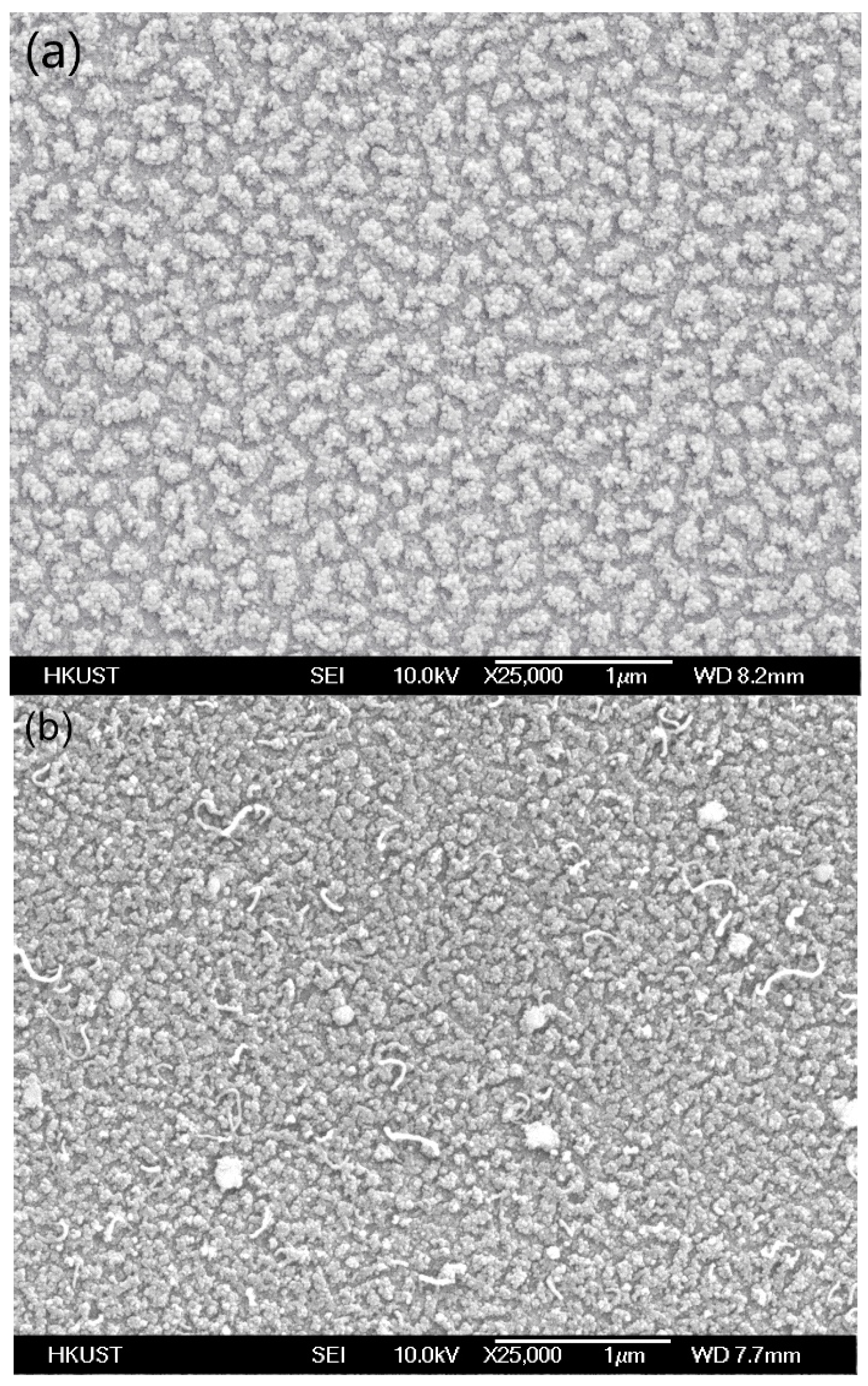
2. Experiment and Results
3. Discussion and Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shulaker, M.M.; Hills, G.; Patil, N.; Wei, H.; Chen, H.Y.; Wong, H.S.P.; Mitra, S. Carbon nanotube computer. Nature 2013, 501, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Kreupl, K.; Graham, A.P.; Libau, M.; Duesberg, G.S.; Seidel, R.; Unger, E. Carbon nanotube for interconnect applications; IEEE IEDM: San Francisco, CA, USA, 2004. [Google Scholar]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef] [Green Version]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bechas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Awano, Y.; Sato, S.; Nihei, M.; Sakai, T.; Ohno, Y.; Mizutani, T. Carbon nanotubes for VLSI: Interconnect and transistor applications. Proc. IEEE 2010, 98, 2015–2031. [Google Scholar] [CrossRef]
- Nihei, M.; Kawabata, A.; Kondo, D.Y.; Horibe, M.; Sato, S.; Awano, Y. Electrical properties of carbon nanotube bundles for future via interconnects. Jpn. J. Appl. Phys. 2005, 44, 1626–1628. [Google Scholar] [CrossRef]
- Ngo, Q.; Cassell, A.M.; Li, J.; Krishnan, S.; Meyyappan, M.; Yang, C.Y. Characteristics of aligned carbon nanotufibers for interconnect via applications. IEEE EDL 2006, 27, 221–224. [Google Scholar] [CrossRef]
- Li, S.W.; Raju, S.; Zhou, C.J.; Chan, M.S. Carbon nanotube contact plug on silicide for CMOS compatible interconnect. IEEE Electron Device Lett. 2016, 36, 793–796. [Google Scholar] [CrossRef]
- Liao, A.D.; Araujo, P.T.; Xu, R.; Dresselhaus, M.S. Carbon nanotube network-silicon oxide non-volatile switches. Nat. Commun. 2014, 5, 5673. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Wu, Y.; Takei, K.; Chen, H.Y.; Yu, S.M.; Chan, P.C.H.; Javey, A.; Wong, H.S.P. Resistive switching of carbon-based RRAM with CNT electrodes for ultra-dense memory; IEEE IEDM: San Francisco, CA, USA, 2010. [Google Scholar]
- Wu, Y.; Chai, Y.; Chen, H.Y.; Yu, S.M.; Wong, H.S.P. Resistive switching AlOx-based memory with CNT electrode for ultra-low switching current and high density memory application; VLSI: Honolulu, HI, USA, 2010; Volume 87. [Google Scholar]
- Mendoza, F.; Hernandez, D.M.; Makarov, V.; Febus, E.; Weiner, B.R.; Morell, G. Room temperature gas sensor based on tin dioxide-carbon nanotubes composite films. Sens. Actuators B 2014, 190, 227–233. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef]
- Bell, M.S.; Teo, K.; Li, J.; Larceda, R.G.; Milne, W.I.; Hash, D.B.; Meyyappan, M. Carbon nanotubes by plasma enhanced chemical vapor deposition. Pure Appl. Chem. 2006, 78, 1117–1125. [Google Scholar] [CrossRef]
- Lee, Y.T.; Park, J.; Choi, Y.S.; Ryu, H.; Lee, H.J. Temperature-dependent growth of vertically aligned carbon nanotubes in the range 800–1100 °C. J. Phys. Chem. B 2002, 106, 7614–7618. [Google Scholar] [CrossRef]
- Vollebregt, S.; Ishihara, R.; Tichelaar, F.D.; Hou, Y.; Beenakker, C.I.M. Influence of the growth temperature on the first and second-order Raman band ratios and widths of carbon nanotubes and fibers. Carbon 2012, 50, 3542–3554. [Google Scholar] [CrossRef]
- Yang, J.; Esconjauregui, S.; Robertson, A.W.; Guo, Y.; Hallam, T.; Sugime, H.; Zhong, G.; Duesberg, G.S. Robertson, Growth of high-density carbon nanotube forests on conductive TiSiN supports. Appl. Phys. Lett. 2015, 106, 083108. [Google Scholar] [CrossRef]
- Sugime, H.; Esconjauregui, S.; Yang, J.W.; Arsie, L.D.; Oliver, R.A.; Bhardwaj, S.; Cepek, C.; Robertson, J. Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports. Appl. Phys. Lett. 2013, 103, 073116. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, F.; Allen, C.S.; Bayer, B.C.; Hofmann, S.; Hickey, B.J.; Cott, D.; Zhong, G.; Robertson, J. Growth of vertically-aligned carbon nanotube forests on conductive cobalt disilicide support. J. Appl. Phys. 2010, 108, 024311. [Google Scholar] [CrossRef]
- Sugime, H.; Esconjauregui, S.; Arsie, L.D.; Yang, J.W.; Robertson, A.W.; Oliver, R.A.; Bhardwaj, S.; Cepek, C.; Robertson, J. Low-temperature growth of carbon nanotube forests consisting of tubes with narrow inner spacing using Co/Al/Mo catalyst on conductive supports ACS. Appl. Mater. Interfaces 2015, 7, 16819–16827. [Google Scholar] [CrossRef] [PubMed]
- Cantoro, M.; Hofmann, S.; Pisana, S.; Scardaci, V.; Parvez, A.; Ducati, C.; Ferrari, A.C.; Blackburn, A.M.; Wang, K.Y.; Robertson, J. Catalytic chemical vapor deposition of single-wall carbon nanotubes at low temperatures. Nano Lett. 2006, 6, 1107–1112. [Google Scholar] [CrossRef]
- Wirth, C.T.; Bayer, B.C.; Gamalski, A.D.; Esconjauregui, S.; Weatherup, R.S.; Ducati, C.; Baehtz, C.; Robertson, J.; Hofmann, S. The phase of iron catalyst nanoparticles during carbon nanotube growth. Chem. Mater. 2012, 24, 4633–4640. [Google Scholar] [CrossRef]
- Reilly, P.T.A.; Whitten, W.B. The role of free radical condensates in the production of carbon nanotubes during the hydrocarbon CVD process. Carbon 2006, 44, 1653–1660. [Google Scholar] [CrossRef]
- Vollebregt, S.; Tichelaar, F.D.; Schellevis, H.; Beenakker, C.I.M.; Ishihara, R. Carbon nanotube vertical interconnects fabricated at temperatures as low as 350 °C. Carbon 2014, 71, 249–256. [Google Scholar] [CrossRef]
- Azam, M.A.; Fujiwara, A.; Shimoda, T. Thermally oxidized aluminum as catalyst-support layer for vertically aligned single-walled carbon nanotube growth using ethanol. Appl. Surf. Sci. 2011, 258, 873–882. [Google Scholar] [CrossRef]
- Mattevi, C.; Wirth, C.T.; Hofmann, S.; Blume, R.; Cantoro, M.; Ducati, C.; Cepek, C.; Gericke, A.K.; Milne, S.; Cudia, C.C.; et al. In-situ X-ray photoelectron spectroscopy study of catalyst−support interactions and growth of carbon nanotube forests. J. Phys. Chem. C 2008, 112, 12207–12213. [Google Scholar] [CrossRef]
- Helveg, S.; Cartes, C.L.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Nielsen, J.R.; Pedersen, F.A.; Norskov, J.K. Atomic-scale imaging of carbon nanofiber growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef] [PubMed]



| Catalysts | Growth Rate (nm/min) | Density (tubes/cm2) |
|---|---|---|
| Ni (2 nm) | 800 | 1 × 1011 |
| Ni–Al–Ni (1 nm–0.5 nm–1 nm) | 1200 | 5 × 1011 |
| Ni–Al–Ni (1 nm–1 nm–1 nm) | 200 | 1 × 109 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Ahmed, Z.; Ma, Z.; Zhou, C.; Zhang, L.; Chan, M. Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate. Nanomaterials 2019, 9, 473. https://doi.org/10.3390/nano9030473
Xiao Y, Ahmed Z, Ma Z, Zhou C, Zhang L, Chan M. Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate. Nanomaterials. 2019; 9(3):473. https://doi.org/10.3390/nano9030473
Chicago/Turabian StyleXiao, Ying, Zubair Ahmed, Zichao Ma, Changjian Zhou, Lining Zhang, and Mansun Chan. 2019. "Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate" Nanomaterials 9, no. 3: 473. https://doi.org/10.3390/nano9030473
APA StyleXiao, Y., Ahmed, Z., Ma, Z., Zhou, C., Zhang, L., & Chan, M. (2019). Low Temperature Synthesis of High-Density Carbon Nanotubes on Insulating Substrate. Nanomaterials, 9(3), 473. https://doi.org/10.3390/nano9030473





