Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis and Characterization of AAILs
2.3. Solvent Extraction Assisted by AAILs
2.4. Interfacial Tension Measurements
2.5. Computational Simulation
3. Results and Discussion
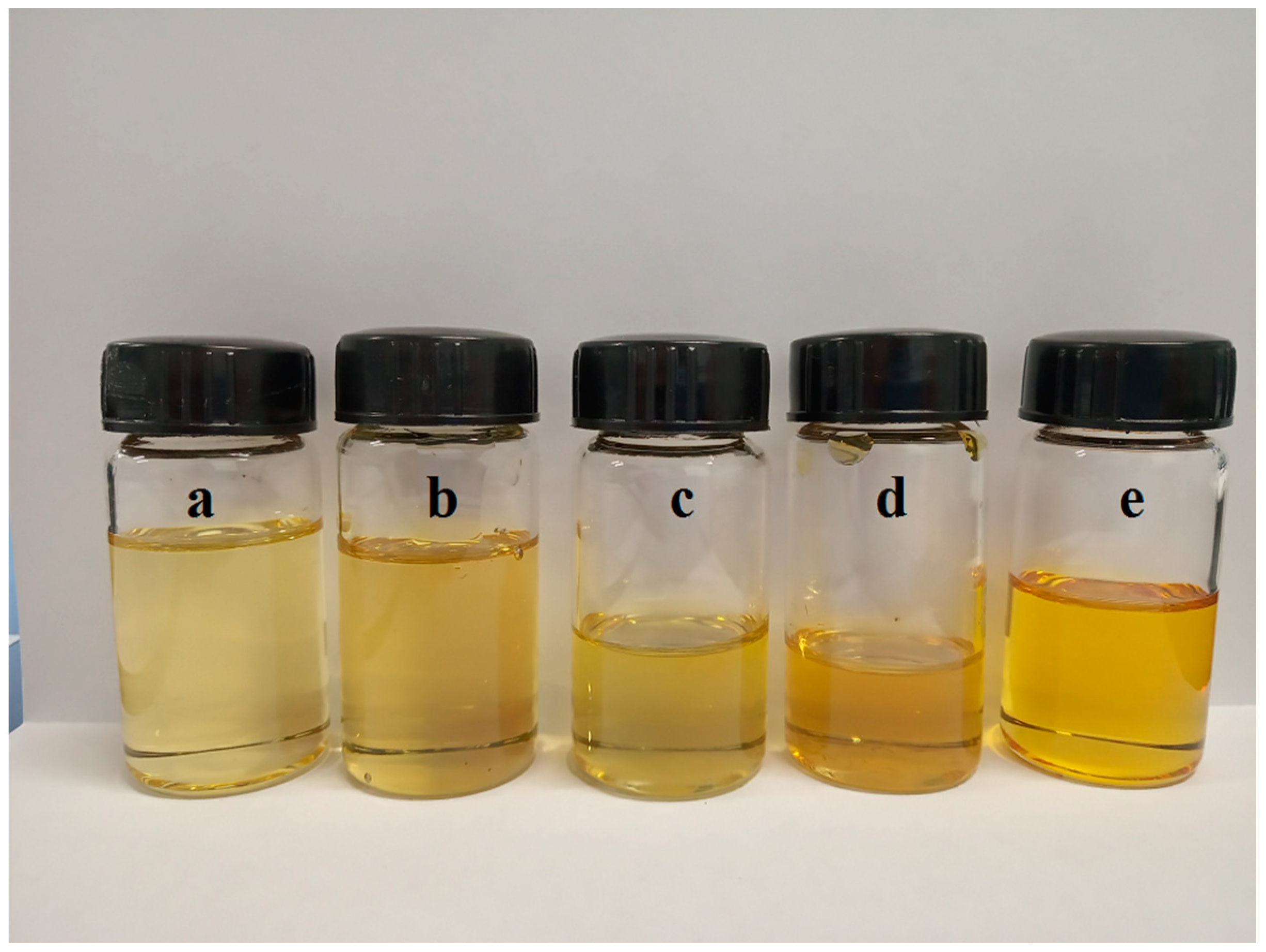
3.1. Characterizations of AAILs
3.2. Application in Carbonate Asphalt Rocks Separation
3.3. Computational Simulation for AAIL-Assisted Solvent Extraction
3.4. The Selection of AAIL for Enhancing Oil Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef]
- Czarnecki, J.; Radoev, B.; Schramm, L.L.; Slavchev, R. On the nature of Athabasca Oil Sands. Adv. Colloid Interface Sci. 2005, 114–115, 53–60. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Wu, G.; Sun, W.; Li, H.; Sui, H. Operational Parameters, Evaluation Methods, And Fundamental Mechanisms: Aspects of Nonaqueous Extraction of Bitumen from Oil Sands. Energy Fuels 2012, 26, 3553–3563. [Google Scholar] [CrossRef]
- Sui, H.; Ma, G.; He, L.; Zhang, Z.; Li, X. Recovery of Heavy Hydrocarbons from Indonesian Carbonate Asphalt Rocks. Part 1: Solvent Extraction, Particle Sedimentation, and Solvent Recycling. Energy Fuels 2016, 30, 9242–9249. [Google Scholar] [CrossRef]
- Nikakhtari, H.; Vagi, L.; Choi, P.; Liu, Q.; Gray, M.R. Solvent screening for non-aqueous extraction of Alberta oil sands. Can. J. Chem. Eng. 2013, 91, 1153–1160. [Google Scholar] [CrossRef]
- Pal, K.; Nogueira Branco, L.D.; Heintz, A.; Choi, P.; Liu, Q.; Seidl, P.R.; Gray, M.R. Performance of Solvent Mixtures for Non-aqueous Extraction of Alberta Oil Sands. Energy Fuels 2015, 29, 2261–2267. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Sui, H.; Jain, A.; He, L. A hybrid process for oil-solid separation by a novel multifunctional switchable solvent. Fuel 2018, 221, 303–310. [Google Scholar] [CrossRef]
- Painter, P.; Williams, P.; Mannebach, E. Recovery of Bitumen from Oil or Tar Sands Using Ionic Liquids. Energy Fuels 2009, 24, 1094–1098. [Google Scholar] [CrossRef]
- Painter, P.; Williams, P.; Lupinsky, A. Recovery of Bitumen from Utah Tar Sands Using Ionic Liquids. Energy Fuels 2010, 24, 5081–5088. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Wu, G.; He, L.; Li, H.; Sui, H. Ionic Liquid Enhanced Solvent Extraction for Bitumen Recovery from Oil Sands. Energy Fuels 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; He, L.; Sui, H.; Yin, W. Ionic Liquid-Assisted Solvent Extraction for Unconventional Oil Recovery: Computational Simulation and Experimental Tests. Energy Fuels 2016, 30, 7074–7081. [Google Scholar] [CrossRef]
- Qing, W.; Chunxia, J.; Qianqian, J.; Yin, W.; Wu, D. Combustion characteristics of Indonesian oil sands. Fuel Process. Technol. 2012, 99, 110–114. [Google Scholar] [CrossRef]
- Nie, F.; He, D.; Guan, J.; Bao, H.; Zhang, K.; Meng, T.; Zhang, Q. Influence of Temperature on the Product Distribution during the Fast Pyrolysis of Indonesian Oil Sands and the Relationships of the Products to the Oil Sand Organic Structure. Energy Fuels 2017, 31, 1318–1328. [Google Scholar] [CrossRef]
- Zhang, Z.; Bei, H.; Li, H.; Li, X.; Gao, X. Understanding the Co-Pyrolysis Behavior of Indonesian Oil Sands and Corn Straw. Energy Fuels 2017, 31, 2538–2547. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Li, H.; Li, X.; Gao, X. Understanding the pyrolysis progress physical characteristics of Indonesian oil sands by visual experimental investigation. Fuel 2018, 216, 29–35. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef]
- Kalb, R.S.; Damm, M.; Verevkin, S.P. Carbonate Based Ionic Liquid Synthesis (CBILS (R)): Development of the continuous flow method for preparation of ultra-pure ionic liquids. React. Chem. Eng. 2017, 2, 432–436. [Google Scholar] [CrossRef]
- Tao, G.H.; He, L.; Sun, N.; Kou, Y. New generation ionic liquids: Cations derived from amino acids. Chem. Commun 2005, 0, 3562–3564. [Google Scholar] [CrossRef]
- Tao, G.H.; He, L.; Liu, W.S.; Xu, L.; Xiong, W.; Wang, T.; Kou, Y. Preparation, characterization and application of amino acid-based green ionic liquids. Green Chem. 2006, 8, 639–646. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, A.M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Fukumoto, K.; Ohno, H. Design and synthesis of hydrophobic and chiral anions from amino acids as precursor for functional ionic liquids. ChemInform 2006, 37, 3081–3083. [Google Scholar] [CrossRef]
- Liu, Q.P.; Hou, X.D.; Li, N.; Zong, M.H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Wang, J.; Sui, H.; He, L.; Li, X. Synthesis and application of amino acid ionic liquid-based deep eutectic solvents for oil-carbonate mineral separation. Chem. Eng. Sci. 2018, 181, 264–271. [Google Scholar] [CrossRef]
- Sui, H.; Ma, G.; Yuan, Y.; Li, Q.; He, L.; Wang, Y.; Li, X. Bitumen-silica interactions in the presence of hydrophilic ionic liquids. Fuel 2018, 233, 860–866. [Google Scholar] [CrossRef]
- Bai, Y.; Sui, H.; Liu, X.; He, L.; Li, X.; Thormann, E. Effects of the N, O, and S heteroatoms on the adsorption and desorption of asphaltenes on silica surface: A molecular dynamics simulation. Fuel 2019, 240, 252–261. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Sui, H.; He, L. Understanding the Liberation of Asphaltenes on the Muscovite Surface. Energy Fuels 2017, 31, 1174–1181. [Google Scholar] [CrossRef]
- De Castro, M.D.L.; Garcia-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Wu, G.Z.; He, L.; Chen, D.Y. Sorption and distribution of asphaltene, resin, aromatic and saturate fractions of heavy crude oil on quartz surface: Molecular dynamic simulation. Chemosphere 2013, 92, 1465–1471. [Google Scholar] [CrossRef]
- Rogel, E. Asphaltene aggregation: A molecular thermodynamic approach. Langmuir 2002, 18, 1928–1937. [Google Scholar] [CrossRef]
- Sjoblom, J.; Simon, S.; Xu, Z. Model molecules mimicking asphaltenes. Adv. Colloid Interface Sci. 2015, 218, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstraete, J.J.; Schnongs, P.; Dulot, H.; Hudebine, D. Molecular reconstruction of heavy petroleum residue fractions. Chem. Eng. Sci. 2010, 65, 304–312. [Google Scholar] [CrossRef]
- Ni, X.; Choi, P. Wetting Behavior of Nanoscale Thin Films of Selected Organic Compounds and Water on Model Basal Surfaces of Kaolinite. J. Phys. Chem. C 2012, 116, 26275–26283. [Google Scholar] [CrossRef]








| Name | Structure | ρ a (g/cm−3) | pI b | Tm c (°C) |
|---|---|---|---|---|
| Glycine (Gly) |  | 1.595 | 5.97 | 240 |
| Histidine(His) |  | 1.309 | 7.59 | 282 |
| Serine (Ser) |  | 1.530 | 5.68 | 240 |
| Proline (Pro) |  | 1.350 | 6.30 | 228 |
| Phenylalanine (Phe) |  | 1.290 | 5.48 | 283 |
| Tg (°C ) | Td (°C) | Viscosity (mPa·s) a | Density (g/cm−3) | Surfase Tension (mN/m) b | |
|---|---|---|---|---|---|
| ChGly | −83.17 | 138.57 | 32.08 | 1.140 | 61.81 |
| ChHis | −51.15 | 177.75 | 570.3 | 1.129 | 59.22 |
| ChSer | −62.53 | 184.56 | 352.5 | 1.156 | 57.66 |
| ChPro | −69.78 | 165.08 | 272.8 | 1.111 | 55.86 |
| ChPhe | −49.71 | 163.35 | 443.8 | 1.121 | 56.01 |
| ChGly | ChHis | ChSer | ChPro | ChPhe | |
|---|---|---|---|---|---|
| Surface tension of AAIL (mN/m) | 61.81 | 59.22 | 57.66 | 55.86 | 56.01 |
| Interfacial tension of AAIL-toluene interface (mN/m) | 19.08 | 8.48 | 15.92 | 9.04 | 6.43 |
| ChGly-Toluene | ChHis-Toluene | ChSer-Toluene | ChPro-Toluene | ChPhe-Toluene | Toluene | |
|---|---|---|---|---|---|---|
| Solids entrained (mg/mL) | 3.00 ± 0.38 | 1.41 ± 0.29 | 2.25 ± 0.31 | 2.63 ± 0.49 | 1.13 ± 0.29 | 13.17 ± 0.42 |
| Saturates | Aromatics | Resins | Asphaltenes | Toluene | |
|---|---|---|---|---|---|
| ChGly | 7.01 × 10−5 | 2.80 × 10−4 | 2.96 × 10−7 | 2.26 × 10−3 | 7.16 × 10−2 |
| ChHis | 7.75 × 10−7 | 4.80 × 10−7 | 3.55 × 10−11 | 3.13 × 10−6 | 3.86 × 10−2 |
| ChSer | 1.04 × 10−5 | 1.29 × 10−5 | 5.93 × 10−9 | 8.59 × 10−5 | 5.06 × 10−2 |
| ChPro | 3.03 × 10−6 | 1.27 × 10−6 | 4.03 × 10−10 | 6.03 × 10−6 | 4.78 × 10−2 |
| ChPhe | 3.25 × 10−6 | 2.03 × 10−6 | 4.53 × 10−10 | 1.03 × 10−5 | 6.31 × 10−2 |
| Etotal (kcal/mol) | Eadsorbate (kcal/mol) | Esurface (kcal/mol) | Einteraction (kcal/mol) | ||||
|---|---|---|---|---|---|---|---|
| Eelctrostatic | Evan der Walls | Eelctrostatic | Evan der Walls | Eelctrostatic | Evan der Walls | ||
| ChGly-calcite | 23460.41 | 275.89 | 25114.15 | −1929.63 | |||
| −15706.20 | 39170.22 | 270.76 | 5.21 | −14027.70 | 39144.45 | ||
| ChHis-calcite | 23463.15 | 271.39 | 25211.37 | −2019.61 | |||
| −15710.42 | 39177.45 | 263.04 | 8.513 | −13930.65 | 39144.45 | ||
| ChSer-calcite | 23454.89 | 292.02 | 25112.27 | −1949.39 | |||
| −15729.79 | 39188.45 | 283.86 | 8.26 | −14029.58 | 39144.45 | ||
| ChPro-calcite | 23469.95 | 266.68 | 25147.58 | −1944.31 | |||
| −15725.47 | 39199.21 | 261.38 | 5.42 | −13994.33 | 39144.45 | ||
| ChPhe-calcite | 23500.08 | 281.42 | 25226.52 | −2007.85 | |||
| −15673.39 | 39177.41 | 270.49 | 11.12 | −13915.53 | 39144.45 | ||
| Asphaltene-calcite | 25406.75 | 39.18 | 25459.07 | −91.51 | |||
| −13718.42 | 39131.62 | 37.11 | 3.32 | −13683.35 | 39144.45 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kang, N.; Zhou, J.; Li, X.; He, L.; Sui, H. Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials 2019, 9, 504. https://doi.org/10.3390/nano9040504
Zhang Z, Kang N, Zhou J, Li X, He L, Sui H. Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials. 2019; 9(4):504. https://doi.org/10.3390/nano9040504
Chicago/Turabian StyleZhang, Zisheng, Ning Kang, Jingjing Zhou, Xingang Li, Lin He, and Hong Sui. 2019. "Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks" Nanomaterials 9, no. 4: 504. https://doi.org/10.3390/nano9040504
APA StyleZhang, Z., Kang, N., Zhou, J., Li, X., He, L., & Sui, H. (2019). Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials, 9(4), 504. https://doi.org/10.3390/nano9040504





