Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Photocatalysts
2.2. Photocatalytic Experiments
2.3. Preparation of the Photocatalitic Membrane and Fouling Tests
3. Results and Discussion
3.1. Characterization of Photocatalysts
3.2. Adsorption Isotherms
3.3. Photocatalytic Activity
3.3.1. Effect of the Initial HA Concentration
3.3.2. Effect of the Initial pH
3.3.3. Effect of Catalyst Dosage
3.4. Stability of Ce-ZrO2 Photocatalyst
3.5. Spectral and TOC Changes in Treated HA Solutions
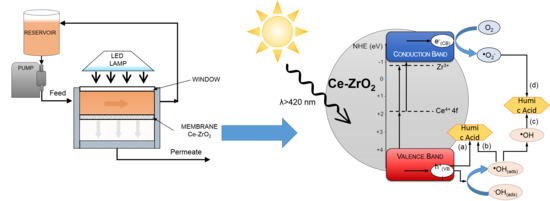
3.6. Effect of Scavengers—Photocatalytic Mechanism
3.7. Filtration Experiments with the Photocatalytic Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Hydrophilic composite membranes for simultaneous separation and photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2013, 111, 9–19. [Google Scholar] [CrossRef]
- Choi, H.G.; Son, M.; Yoon, S.H.; Celik, E.; Kang, S.; Park, H.; Park, C.H.; Choi, H. Alginate fouling reduction of functionalized carbon nanotube blended cellulose acetate membrane in forward osmosis. Chemosphere 2015, 136, 204–210. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, B.H. Pilot Study Analysis of Three Different Processes in Drinking Water Treatment. Environ. Eng. Res. 2011, 16, 237–242. [Google Scholar] [CrossRef]
- Duong, H.C.; Duke, M.; Gray, S.; Cooper, P.; Nghiem, L.D. Membrane scaling and prevention techniques during seawater desalination by air gap membrane distillation. Desalination 2016, 397, 92–100. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic Acid Fouling during Ultrafiltration. Environ. Sci. Technol. 2000, 34, 5043–5050. [Google Scholar] [CrossRef]
- Charfi, A.; Jang, H.; Kim, J. Membrane fouling by sodium alginate in high salinity conditions to simulate biofouling during seawater desalination. Bioresour. Technol. 2017, 240, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, Y.; Yu, H.; Kang, G.; Jie, X.; Liu, Z.; Yuan, Q. A novel composite nanofiltration membrane prepared with PHGH and TMC by interfacial polymerization. J. Memb. Sci. 2014, 466, 82–91. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, Y.; Wang, J.; Wang, S. Surface modification of NF membrane with zwitterionic polymer to improve anti-biofouling property. J. Memb. Sci. 2016, 514, 407–417. [Google Scholar] [CrossRef]
- Mustafa, G.; Wyns, K.; Vandezande, P.; Buekenhoudt, A.; Meynen, V. Novel grafting method efficiently decreases irreversible fouling of ceramic nanofiltration membranes. J. Memb. Sci. 2014, 470, 369–377. [Google Scholar] [CrossRef]
- Goosen, M.F.A.; Sablani, S.S.; Al-Hinai, H.; Al-Obeidani, S.; Al-Belushi, R.; Jackson, D. Fouling of Reverse Osmosis and Ultrafiltration Membranes: A Critical Review. Sep. Sci. Technol. 2005, 39, 2261–2297. [Google Scholar] [CrossRef]
- de Lara, R.; Benavente, J. Use of hydrodynamic and electrical measurements to determine protein fouling mechanisms for microfiltration membranes with different structures and materials. Sep. Purif. Technol. 2009, 66, 517–524. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Sun, X.; Wu, J.; Chen, Z.; Su, X.; Hinds, B.J. Fouling Characteristics and Electrochemical Recovery of Carbon Nanotube Membranes. Adv. Funct. Mater. 2013, 23, 1500–1506. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Mänttäri, M.; Nyström, M. Drawbacks of applying nanofiltration and how to avoid them: A review. Sep. Purif. Technol. 2008, 63, 251–263. [Google Scholar] [CrossRef]
- Kim, E.S.; Yu, Q.; Deng, B. Plasma surface modification of nanofiltration (NF) thin-film composite (TFC) membranes to improve anti organic fouling. Appl. Surf. Sci. 2011, 257, 9863–9871. [Google Scholar] [CrossRef]
- Asatekin, A.; Kang, S.; Elimelech, M.; Mayes, A.M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly(ethylene oxide) comb copolymer additives. J. Memb. Sci. 2007, 298, 136–146. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High performance and antifouling vertically aligned carbon nanotube membrane for water purification. J. Memb. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic Thin-Film Composite Forward Osmosis Membranes for Organic Fouling Control: Fouling Behavior and Antifouling Mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, K.; Davies, C.; Wang, H. Carbon Nanotube/Alumina/Polyethersulfone Hybrid Hollow Fiber Membranes with Enhanced Mechanical and Anti-Fouling Properties. Nanomaterials 2015, 5, 1366–1378. [Google Scholar] [CrossRef]
- Rao, G.; Zhang, Q.; Zhao, H.; Chen, J.; Li, Y. Novel titanium dioxide/iron (III) oxide/graphene oxide photocatalytic membrane for enhanced humic acid removal from water. Chem. Eng. J. 2016, 302, 633–640. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Takeuchi, M.; Yamashita, H.; Matsuoka, M.; Anpo, M.; Hirao, T.; Itoh, N.; Iwamoto, N. Photocatalytic decomposition of NO under visible light irradiation on the Cr-ion-implanted TiO2 thin film photocatalyst. Catal. Lett. 2000, 67, 135–137. [Google Scholar] [CrossRef]
- Asahi, R. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic solar water splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Sacco, O.; Vaiano, V.; Sannino, D. Rare earth oxides in zirconium dioxide: How to turn a wide band gap metal oxide into a visible light active photocatalyst. J. Energy Chem. 2017, 26, 270–276. [Google Scholar] [CrossRef]
- Gionco, C.; Battiato, A.; Vittone, E.; Paganini, M.C.; Giamello, E. Structural and spectroscopic properties of high temperature prepared ZrO2-TiO2 mixed oxides. J. Solid State Chem. 2013, 201, 222–228. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Li, L.-L.; Li, Z.-X.; Fang, C.-J.; Duan, W.-T.; Li, X.-G.; Yan, C.-H. Highly ordered mesoporous titania–zirconia photocatalyst for applications in degradation of rhodamine-B and hydrogen evolution. Microporous Mesoporous Mater. 2009, 124, 169–178. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Aflaki, M.; Davar, F. Synthesis, luminescence and photocatalyst properties of zirconia nanosheets by modified Pechini method. J. Mol. Liq. 2016, 221, 1071–1079. [Google Scholar] [CrossRef]
- Reddy, V.R.; Hwang, D.W.; Lee, J.S. Photocatalytic water splitting over ZrO2 prepared by precipitation method. Korean J. Chem. Eng. 2003, 20, 1026–1029. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Burgess, R.; Di Valentin, C.; Pacchioni, G. Cerium-doped zirconium dioxide, a visible-light-sensitive photoactive material of third generation. J. Phys. Chem. Lett. 2014, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Gionco, C.; Paganini, M.C.; Chiesa, M.; Maurelli, S.; Livraghi, S.; Giamello, E. Cerium doped zirconium dioxide as a potential new photocatalytic material. the role of the preparation method on the properties of the material. Appl. Catal. A Gen. 2015, 504, 338–343. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Wang, Y.; Wang, W.; Wang, X.; Bu, Y.; Zhao, J. Adsorption-photodegradation of humic acid in water by using ZnO coupled TiO2/bamboo charcoal under visible light irradiation. J. Hazard. Mater. 2013, 262, 16–24. [Google Scholar] [CrossRef]
- Oskoei, V.; Dehghani, M.H.; Nazmara, S.; Heibati, B.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Removal of humic acid from aqueous solution using UV/ZnO nano-photocatalysis and adsorption. J. Mol. Liq. 2016, 213, 374–380. [Google Scholar] [CrossRef]
- Wang, G. Destruction of humic acid in water by UV light catalyzed oxidation with hydrogen peroxide. Water Res. 2000, 34, 3882–3887. [Google Scholar] [CrossRef]
- Birben, N.C.; Uyguner-Demirel, C.S.; Kavurmaci, S.S.; Gürkan, Y.Y.; Turkten, N.; Cinar, Z.; Bekbolet, M. Application of Fe-doped TiO2 specimens for the solar photocatalytic degradation of humic acid. Catal. Today 2017, 281, 78–84. [Google Scholar] [CrossRef]
- Najm, I.N.; Patania, N.L.; Jacangelo, J.G.; Krasner, S.W. Evaluating surrogates for disinfection by-products. J. Am. Water Works Assoc. 1994, 86, 98–106. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV-vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- García-López, E.; Marcì, G.; Pomilla, F.R.; Paganini, M.C.; Gionco, C.; Giamello, E.; Palmisano, L. ZrO2 Based materials as photocatalysts for 2-propanol oxidation by using UV and solar light irradiation and tests for CO2 reduction. Catal. Today 2018, 313, 100–105. [Google Scholar] [CrossRef]
- Hernández, S.; Gionco, C.; Husak, T.; Castellino, M.; Muñoz-Tabares, J.A.; Tolod, K.R.; Giamello, E.; Paganini, M.C.; Russo, N. Insights Into the Sunlight-Driven Water Oxidation by Ce and Er-Doped ZrO2. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Wang, D.; Guo, L.; Zhen, Y.; Yue, L.; Xue, G.; Fu, F. AgBr quantum dots decorated mesoporous Bi 2 WO 6 architectures with enhanced photocatalytic activities for methylene blue. J. Mater. Chem. A 2014, 2, 11716–11727. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Ranjan Sahu, H.; Ranga Rao, G. Characterization of combustion synthesized zirconia powder by UV-vis, IR and other techniques. Bull. Mater. Sci. 2000, 23, 349–354. [Google Scholar] [CrossRef]
- Gionco, C.; Paganini, M.C.; Giamello, E.; Burgess, R.; Di Valentin, C.; Pacchioni, G. Paramagnetic Defects in Polycrystalline Zirconia: An EPR and DFT Study. Chem. Mater. 2013, 25, 2243–2253. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.F. TiO2 microsphere for the removal of humic acid from water: Complex surface adsorption mechanisms. Sep. Purif. Technol. 2012, 91, 30–37. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Amal, R. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration. Chem. Eng. Sci. 2014, 105, 46–52. [Google Scholar] [CrossRef]
- Spark, K.M.; Wells, J.D.; Johnson, B.B. The interaction of a humic acid with heavy metals. Aust. J. Soil Res. 1997, 35, 89. [Google Scholar] [CrossRef]
- Kipton, H.; Powell, J.; Town, R.M. Solubility and fractionation of humic acid; effect of pH and ionic medium. Anal. Chim. Acta 1992, 267, 47–54. [Google Scholar] [CrossRef]
- Ferro-García, M.A.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Adsorption of Humic Substances on Activated Carbon from Aqueous Solutions and Their Effect on the Removal of Cr(III) Ions. Langmuir 1998, 14, 1880–1886. [Google Scholar] [CrossRef]
- Moussavi, G.; Talebi, S.; Farrokhi, M.; Sabouti, R.M. The investigation of mechanism, kinetic and isotherm of ammonia and humic acid co-adsorption onto natural zeolite. Chem. Eng. J. 2011, 171, 1159–1169. [Google Scholar] [CrossRef]
- Coppola, E.; Iovino, P.; Salvestrini, S.; Capasso, S.; Colella, C. Humic acid sorption properties of calcium-rich derivatives of Neapolitan Yellow Tuff. WIT Trans. Ecol. Environ. 2008, 111, 565–574. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yuan, P.H.; Fan, C.M.; Wang, Y.; Ding, G.Y.; Wang, Y.F. Preparation of zinc titanate nanoparticles and their photocatalytic behaviors in the photodegradation of humic acid in water. Ceram. Int. 2012, 38, 4173–4180. [Google Scholar] [CrossRef]
- Fryxell, G.E.; Cao, G. Environmental Applications of Nanomaterials; Imperial College Press: London, UK, 2012; ISBN 978-1-84816-803-9. [Google Scholar]
- Li, X.Z.; Fan, C.M.; Sun, Y.P. Enhancement of photocatalytic oxidation of humic acid in TiO2 suspensions by increasing cation strength. Chemosphere 2002, 48, 453–460. [Google Scholar] [CrossRef]
- Xue, G.; Liu, H.; Chen, Q.; Hills, C.; Tyrer, M.; Innocent, F. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard. Mater. 2011, 186, 765–772. [Google Scholar] [CrossRef]
- Subagio, D.P.; Srinivasan, M.; Lim, M.; Lim, T.T. Photocatalytic degradation of bisphenol-A by nitrogen-doped TiO2 hollow sphere in a vis-LED photoreactor. Appl. Catal. B Environ. 2010, 95, 414–422. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Guliants, V.V.; Carreon, M.A.; Lin, Y.S. Ordered mesoporous and macroporous inorganic films and membranes. J. Memb. Sci. 2004, 235, 53–72. [Google Scholar] [CrossRef]
- Yan, H.; Chai, L.Y.; Peng, B.; Li, M.; Peng, N.; Hou, D.K. A novel method to recover zinc and iron from zinc leaching residue. Miner. Eng. 2014, 55, 103–110. [Google Scholar] [CrossRef]
- Juez, R.G.; Boffa, V.; Blank, D.H.A.; ten Elshof, J.E. Preparation of self-supporting mesostructured silica thin film membranes as gateable interconnects for microfluidics. J. Memb. Sci. 2008, 323, 347–351. [Google Scholar] [CrossRef]
- Nisticò, R.; Scalarone, D.; Magnacca, G. Sol-gel chemistry, templating and spin-coating deposition: A combined approach to control in a simple way the porosity of inorganic thin films/coatings. Microporous Mesoporous Mater. 2017, 248, 18–29. [Google Scholar] [CrossRef]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere 2002, 46, 1173–1181. [Google Scholar] [CrossRef]
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proença, M.F.; Nogueira, R. Quantification of humic acids in surface water: Effects of divalent cations, pH, and filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Photocatalysis and Photoinduced Hydrophilicity of Various Metal Oxide Thin Films. Chem. Mater. 2002, 14, 2812–2816. [Google Scholar] [CrossRef]
- Kashif, N.; Ouyang, F. Parameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2. J. Environ. Sci. 2009, 21, 527–533. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, R.; Li, G. Degradation of phenol by nanomaterial TiO2 in wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]
- Dehghanian, N.; Ghaedi, M.; Ansari, A.; Ghaedi, A.; Vafaei, A.; Asif, M.; Agarwal, S.; Tyagi, I.; Gupta, V.K. A random forest approach for predicting the removal of Congo red from aqueous solutions by adsorption onto tin sulfide nanoparticles loaded on activated carbon. Desalin. Water Treat. 2016, 57, 9272–9285. [Google Scholar] [CrossRef]
- Khezrianjoo, S.; Revanasiddappa, H.D. Photocatalytic Degradation of Acid Yellow 36 Using Zinc Oxide Photocatalyst in Aqueous Media. J. Catal. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Daneshvar, N.; Rasoulifard, M.H.; Khataee, A.R.; Hosseinzadeh, F. Removal of C.I. Acid Orange 7 from aqueous solution by UV irradiation in the presence of ZnO nanopowder. J. Hazard. Mater. 2007, 143, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Motuzas, J.; Martens, W.; Diniz da Costa, J.C. Ceramic metal oxides with Ni 2+ active phase for the fast degradation of Orange II dye under dark ambiance. Ceram. Int. 2018, 44, 6634–6640. [Google Scholar] [CrossRef]
- Calza, P.; Gionco, C.; Giletta, M.; Kalaboka, M.; Sakkas, V.A.; Albanis, T.; Paganini, M.C. Assessment of the abatement of acelsulfame K using cerium doped ZnO as photocatalyst. J. Hazard. Mater. 2017, 323, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Thandu, M.; Comuzzi, C.; Goi, D. Phototreatment of Water by Organic Photosensitizers and Comparison with Inorganic Semiconductors. Int. J. Photoenergy 2015, 2015, 1–22. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Bai, X.; Lv, L.; Ye, Z.; Huang, X. Nitrogen-doped perovskite-type La2Ti2O7decorated on graphene composites exhibiting efficient photocatalytic activity toward bisphenol A in water. J. Colloid Interface Sci. 2015, 450, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zou, G.; Deng, H. The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation. Catalysts 2018, 8, 272. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Bianco Prevot, A.; Magnacca, G. Revisiting the catalytic activity of a doped SrFeO3 for water pollutants removal: Effect of light and temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor photocatalysis—Past, present, and future outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef] [PubMed]
















| Sample | SBET [m2·g−1] |
|---|---|
| ZrO2-HYD | 44 ± 4 |
| Ce-ZrO2-HYD | 49 ± 5 |
| ZrO2-SG | 150 ± 15 |
| Ce-ZrO2-SG | 70 ± 7 |
| Sample | Langmuir Adsorption Model | ||
|---|---|---|---|
| qe [mg·g−1] | kL | R2 | |
| Ce-ZrO2-HYD | 9.3 | 0.36 | 0.998 |
| Ce-ZrO2-SG | 12.3 | 0.74 | 0.997 |
| Catalyst | [HA]0 [mg·L−1] | kobs [min−1] | r2 | k [mg·L−1·min−1] | Kads [L·mg−1] |
|---|---|---|---|---|---|
| Ce-ZrO2-HYD | 20 | 0.0023 | 0.96 | 0.058 | 0.24 |
| 10 | 0.0049 | 0.97 | |||
| 5 | 0.0053 | 0.94 | |||
| Ce-ZrO2-SG | 20 | 0.0040 | 0.91 | 0.099 | 0.28 |
| 10 | 0.0097 | 0.96 | |||
| 5 | 0.0091 | 0.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortot Coelho, F.E.; Gionco, C.; Paganini, M.C.; Calza, P.; Magnacca, G. Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light. Nanomaterials 2019, 9, 534. https://doi.org/10.3390/nano9040534
Bortot Coelho FE, Gionco C, Paganini MC, Calza P, Magnacca G. Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light. Nanomaterials. 2019; 9(4):534. https://doi.org/10.3390/nano9040534
Chicago/Turabian StyleBortot Coelho, Fabrício Eduardo, Chiara Gionco, Maria Cristina Paganini, Paola Calza, and Giuliana Magnacca. 2019. "Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light" Nanomaterials 9, no. 4: 534. https://doi.org/10.3390/nano9040534
APA StyleBortot Coelho, F. E., Gionco, C., Paganini, M. C., Calza, P., & Magnacca, G. (2019). Control of Membrane Fouling in Organics Filtration Using Ce-Doped Zirconia and Visible Light. Nanomaterials, 9(4), 534. https://doi.org/10.3390/nano9040534