Biodegradable Redox-Sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amphiphilic Polymers
2.3. Synthesis of AD-P(LA-co-GA)4 Copolymer
2.4. Synthesis of MPEG-SS-COOH and AD-[P(LA-co-GA)-SS-mPEG]4
2.5. Characterization
2.6. Preparation of Self-Assembled Amphiphilic Polymer Micelles
2.7. Critical Micelle Concentration (CMC) Measurement
2.8. Determination of Particle Size and Polydispersity (PDI) of Polymer Micelles
2.9. Preparation of DOX-Loaded Micelles and Observation of Morphology
2.10. In Vitro Release Experiment of Drug-Loaded Micelles
2.11. Cytotoxicity Test
3. Results and Discussion
3.1. Characterization of Polymer 3
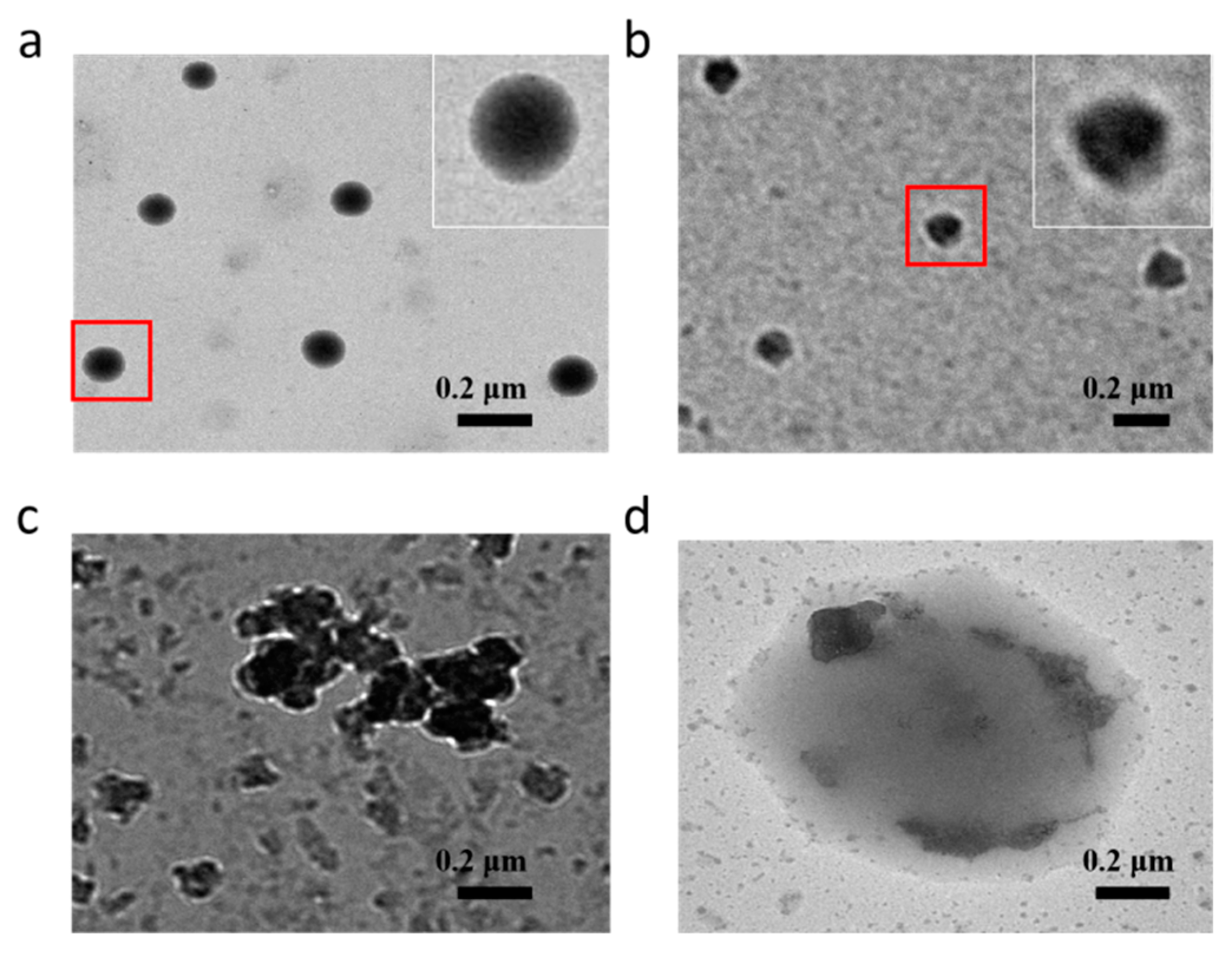
3.2. Self-Assembly of Amphiphilic Polymer 3
3.3. In Vitro Release of DOX from Micelles
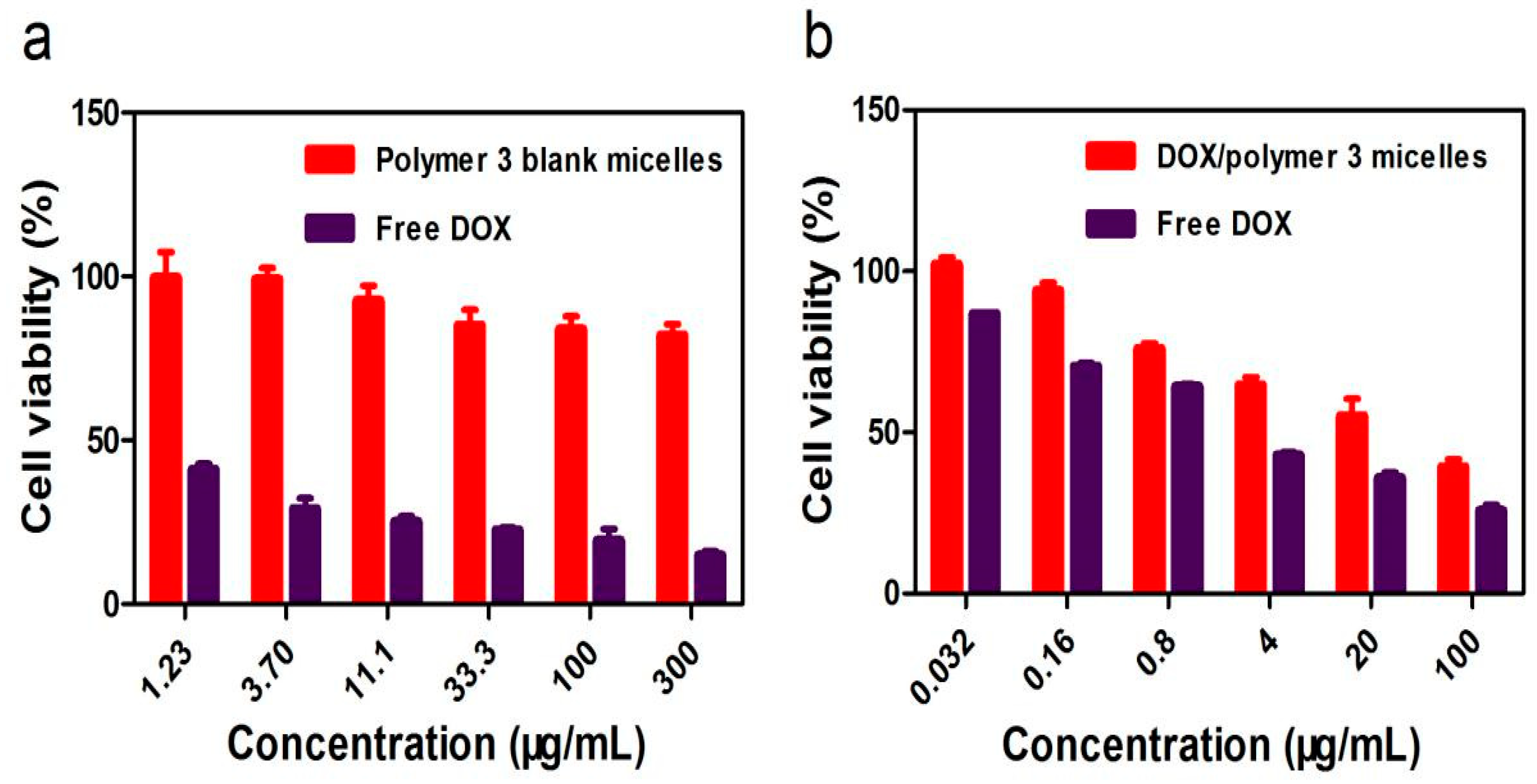
3.4. Cytotoxicity Evaluation and Inhibition of Cancer Cell
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jabri, T.; Imran, M.; Aziz, A.; Rao, K.; Kawish, M.; Irfan, M.; Malik, M.I.; Simjee, S.U.; Arfan, M.; Shah, M.R. Design and Synthesis of Mixed Micellar System for Enhanced Anticancer Efficacy of Paclitaxel through Its Co-delivery with Naringin. Drug Dev. Ind. Pharm. 2018, 703–714. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, B.; Li, Y.-M.; He, F.; Zhuo, R.-X. Folate-conjugated Amphiphilic Block Copolymer Micelle for Targeted and Redox-responsive Delivery of Doxorubicin. J. Biomater. Sci. Polym. Ed. 2018, 29, 92–106. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, S.; Lu, G.; Huang, X. Constructing Well-defined Star Graft Copolymers. Polym. Chem. 2013, 4, 1289–1299. [Google Scholar] [CrossRef]
- Han, J.; Gao, C. Host-guest Supramolecular Chemistry of Dendritic Macromolecules. Curr. Org. Chem. 2011, 15, 2–26. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Xie, J.; Qi, R.; Lu, H.; Leporatti, S.; Chen, J.; Hu, Y. Ph-sensitive Poly(beta-amino ester)s Nanocarriers Facilitate the Inhibition of Drug Resistance in Breast Cancer Cells. Nanomaterials 2018, 8, 952. [Google Scholar] [CrossRef]
- Castro Marin, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thetiot-Laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an Antioxidant Alternative to Sulphites in Oenology: Epr Investigation of Inhibitory Mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef]
- Chen, X.; Yang, B.; Oleszczuk, P.; Gao, Y.; Yuan, X.; Ling, W.; Waigi, M.G. Vanadium Oxide Activates Persulfate for Degradation of Polycyclic Aromatic Hydrocarbons in Aqueous System. Chem. Eng. J. 2019, 364, 79–88. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of Enhanced Permeability and Retention Effect (epr): Nanoparticle-based Precision tools for Targeting of Therapeutic and Diagnostic Agent in Cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar]
- Xi, M.; Jiang, Y. A Ph-responsive Self-fluorescent Polymeric Micelle as a Potential Optical Imaging Probe. Polym. Adv. Technol. 2018, 29, 2002–2009. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, J.; Chu, C.-C. Enhanced Mhc-i Antigen Presentation from the Delivery of Ovalbumin by Light-facilitated Biodegradable Poly (ester amide) s Nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef]
- Qiu, L.Y. In Vitro and in Vivo Degradation Study on Novel Blends Composed of Polyphosphazene and Polyester or Polyanhydride. Polym. Int. 2002, 51, 481–487. [Google Scholar] [CrossRef]
- Guo, Z.H.; Liu, X.F.; Hu, J.S.; Yang, L.Q.; Chen, Z.P. Synthesis and Self-assembled Behavior of Ph-responsive Chiral Liquid Crystal Amphiphilic Copolymers Based on Diosgenyl-functionalized Aliphatic Polycarbonate. Nanomaterials 2017, 7, 169. [Google Scholar] [CrossRef]
- Jeong, G.-W.; Jeong, Y.-I.; Nah, J.-W. Triggered Doxorubicin Release Using Redox-sensitive Hyaluronic Acid-g-stearic Acid Micelles for Targeted Cancer Therapy. Carbohydr. Polym. 2019, 209, 161–171. [Google Scholar] [CrossRef]
- Wang, X.; Lin, W.; Zhang, W.; Li, C.; Sun, T.; Chen, G.; Xie, Z. Amphiphilic Redox-sensitive Nir Bodipy Nanoparticles for Dual-mode Imaging and Photothermal Therapy. J. Colloid Interface Sci. 2019, 536, 208–214. [Google Scholar] [CrossRef]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual Responsive Micelles Capable of Modulating Mirna-34a to Combat Taxane Resistance in Prostate Cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, D.; Chen, H.; Lim, W.Q.; Phua, F.S.Z.; An, G.; Yang, P.; Zhao, Y. Reduction-sensitive Fluorescence Enhanced Polymeric Prodrug Nanoparticles for Combinational Photothermal-chemotherapy. Biomaterials 2018, 163, 14–24. [Google Scholar] [CrossRef]
- Yang, H.; Guo, J.; Tong, R.; Yang, C.; Chen, J.-K. Ph-sensitive Micelles Based on Star Copolymer Ad-(pcl-b-pdeaema-b-ppegma) (4) for Controlled Drug Delivery. Polymers 2018, 10, 443. [Google Scholar] [CrossRef]
- Han, S.; Cheng, Q.; Wu, Y.; Zhou, J.; Long, X.; Wei, T.; Huang, Y.; Zheng, S.; Zhang, J.; Deng, L.; et al. Effects of Hydrophobic Core Components in Amphiphilic Pdmaema Nanoparticles on Sirna Delivery. Biomaterials 2015, 48, 45–55. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, Q.; Zhai, T.; Nie, S.; Luo, J.; Yan, W. Melt Crystallization Behavior and Crystalline Morphology of Polylactide/poly(epsilon-caprolactone) Blends Compatibilized by Lactide-caprolactone Copolymer. Polymers 2018, 10, 1181. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Y.; Wang, W.; Jiang, L.; Dan, Y. Synthesis and Properties of Partially Biodegradable Fluorinated Polyacrylate: Poly (l-lactide)-co-poly(hexafluorobutyl acrylate) Copolymer. Mater. Des. 2019, 162, 285–292. [Google Scholar] [CrossRef]
- Bhayo, A.M.; Abdul-Karim, R.; Musharraf, S.G.; Malik, M.I. Synthesis and Characterization of 4-arm Star-shaped Amphiphilic Block Copolymers Consisting of Poly (ethylene oxide) and Poly (epsilon-caprolactone). RSC Adv. 2018, 8, 28569–28580. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yan, G.; Fu, S.; Tang, R. Ph-sensitive Nanogels with Ortho Ester Linkages Prepared via Thiol-ene Click Chemistry for Efficient Intracellular Drug Release. J. Colloid Interface Sci. 2017, 508, 282–290. [Google Scholar] [CrossRef]
- Fu, S.-Q.; Guo, J.-W.; Zhu, D.-Y.; Yang, Z.; Yang, C.-F.; Xian, J.-X.; Li, X. Novel Halogen-free Flame Retardants Based on Adamantane for Polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Chen, G.; Liu, S.; Ji, N.; Ding, H.; Fu, J. Preparation of Phosphotungstic Acid Based Poly(ionic liquid) and Its Application to Esterification of Palmitic Acid. Renew. Energy 2019, 133, 317–324. [Google Scholar] [CrossRef]
- Halayqa, M.; Zawadzki, M.; Domanska, U.; Plichta, A. Polymer-ionic-liquid Pharmaceutical Conjugates as Drug Delivery Systems. J. Mol. Struct. 2019, 1180, 573–584. [Google Scholar] [CrossRef]
- Fan, X.; Win, K.Y.; Hu, Z.; Loh, X.J.; Li, Z. Precise Synthesis of Ps-pla Janus Star-like Copolymer. Macromol. Rapid Commun. 2019, 40, e1800217. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Ottone, M.; Freitas, M.d.S.; Lima, C.D.; Nelson, D.L.; Clososki, G.C.; Caires, F.J.; Klein, S.I.; Hurtado, G.R. Synthesis of Phenyl Esters Using Sio2-so3h Catalyst in Conventional Heating and Microwave-irradiated Esterification Processes. J. Nanosci. Nanotechnol. 2019, 19, 3663–3668. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Zhu, F.; Ge, M.; Liang, G. Sensitive and Rapid Detection of Aliphatic Amines in Water Using Self-stabilized Micelles of Fluorescent Block Copolymers. J. Hazard. Mater. 2019, 368, 630–637. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, Q.; Zagho, M.M.; Zhang, J.; Elzatahry, A.A.; Jiang, Y.; Deng, Y. Core-shell Magnetic Mesoporous Silica Microspheres with Large Mesopores for Enzyme Immobilization in Biocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 10356–10363. [Google Scholar] [CrossRef]
- Lin, W.; Yang, C.; Xue, Z.; Huang, Y.; Luo, H.; Zu, X.; Zhang, L.; Yi, G. Controlled Construction of Gold Nanoparticles in Situ from Beta-cyclodextrin Based Unimolecular Micelles for in Vitro Computed Tomography. J. Colloid Interface Sci. 2018, 528, 135–144. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Liu, Y.; Xiao, J.; Chen, J.; Zhang, L.; Guo, J.; Lin, W. Delivery of Anticancer Drug Using Ph-sensitive Micelles from Triblock Copolymer Mpeg-b-pbae-b-pla. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Geng, S.; Wang, Y.; Lv, Y.; Wang, H.; Liu, B.; Liang, G. The Interaction Mechanism of Oligopeptides Containing Aromatic rings with Beta-cyclodextrin and Its Derivatives. Food Chem. 2019, 286, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M.A.; Lee, J.E.; Choi, S.S. Structural Insights into the Binding Behavior of Isoflavonoid Glabridin with Human Serum Albumin. Food Hydrocoll. 2019, 91, 290–300. [Google Scholar] [CrossRef]
- Jermsuntiea, W.; Aki, T.; Toyoura, R.; Iwashita, K.; Kawamoto, S.; Ono, K. Purification and Characterization of Intracellular Lipase from the Polyunsaturated Fatty Acid-producing Fungus Mortierella Alliacea. New Biotechnol. 2011, 28, 158–164. [Google Scholar] [CrossRef] [PubMed]







| Sample | Mn,NMR 1 | Mn,GPC 2 | Mw/Mn |
|---|---|---|---|
| AD-(LA60-co-GA20)4 | 11062 | 9789 | 1.39 |
| Polymer 1 | 19586 | 16367 | 1.44 |
| PT-(LA75-co-GA25)4 | 14254 | 11277 | 1.23 |
| Polymer 2 | 20685 | 18504 | 1.21 |
| AD-(LA75-co-GA25)4 | 14138 | 12600 | 1.48 |
| Polymer 3 | 21652 | 19400 | 1.38 |
| Micelle | DOX (mg)/polymer (mg) | DLE (%) | DEE (%) | Size (nm) 1 | PDI |
|---|---|---|---|---|---|
| DOX/polymer 1 | 10/50 | 6.66 | 39.7 | 132 | 0.123 |
| DOX/Polymer 2 | 10/50 | 8.94 | 49.1 | 174 | 0.118 |
| DOX/Polymer 3 | 10/50 | 10.39 | 58.1 | 196 | 0.138 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Guo, J.-W.; Wen, W.-Q.; Chen, J.-K. Biodegradable Redox-Sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery. Nanomaterials 2019, 9, 547. https://doi.org/10.3390/nano9040547
Li M, Guo J-W, Wen W-Q, Chen J-K. Biodegradable Redox-Sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery. Nanomaterials. 2019; 9(4):547. https://doi.org/10.3390/nano9040547
Chicago/Turabian StyleLi, Meng, Jian-Wei Guo, Wei-Qiu Wen, and Jem-Kun Chen. 2019. "Biodegradable Redox-Sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery" Nanomaterials 9, no. 4: 547. https://doi.org/10.3390/nano9040547





