Sb2S3@PPy Coaxial Nanorods: A Versatile and Robust Host Material for Reversible Storage of Alkali Metal Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Sb2S3 Nanorods
2.2. Synthesis of Sb2S3@PPy Coaxial Nanorods
2.3. Materials Characterization
2.4. Electrochemical Performance
3. Results and Discussion
3.1. Materials Synthesis and Characterization
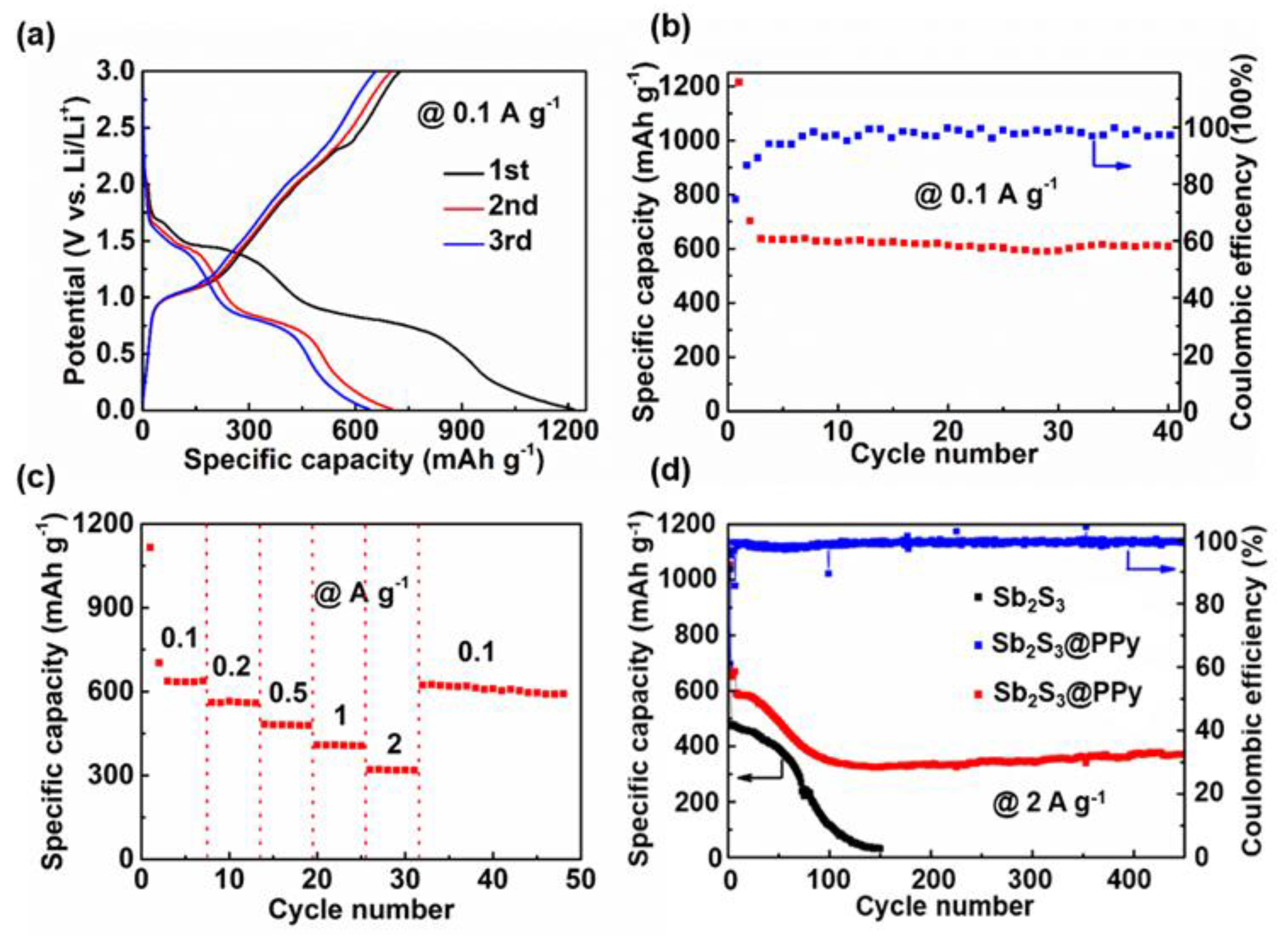
3.2. Lithium Storage Performances
3.3. Sodium Storage Performance
3.4. Potassium Storage Performance
3.5. Alkali Metal Ions Storage Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sang, Y.; Zhao, Z.; Zhao, M.; Hao, P.; Leng, Y.; Liu, H. From UV to near-infrared, WS2 nanosheet: A novel photo catalyst for full solar light spectrum photodegradation. Adv. Mater. 2014, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, L.; Tian, X.; Yan, M.; Yuan, H.; Liao, X.; Meng, J.; Hao, Z.; Mai, L. Carbon-mems-based alternating stacked MoS2@rGO/CNT micro-supercapacitor with high capacitance and energy density. Small 2017, 13, 1700639. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.A.; Rhee, J.H.; Im, S.H.; Lee, Y.H.; Kim, H.; Seok, S.; Nazeeruddin, M.K.; Gratzel, M. High-performance nanostructured inorganic-organic heterojunction solar cells. Nano Lett. 2010, 10, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, X.; Xu, B.; Liu, X.; Kong, Z.; Song, M.; Fu, A.; Li, Y.; Guo, P.; Li, H. Carbon wrapped Ni2S3 nanocrystals anchored on graphene sheets as anode materials for lithium-ion battery and the study on their capacity evolution. Nanomaterials 2018, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Li, F.; Franchetti, P.; Ghanbaja, J.; Mai, L. Fast, green microwave-assisted synthesis of single crystalline Sb2Se3 nanowires towards promising lithium storage. J. Energy Chem. 2018, 30, 27–33. [Google Scholar] [CrossRef]
- Lakshmi, V.; Chen, Y.; Mikhaylov, A.A.; Medvedev, G.A.; Sultana, I.; Rahman, M.M.; Lev, O.; Prikhodchenko, P.V.; Glushenkov, A.M. Nanocrystalline SnS2 coated onto reduced graphene oxide: Demonstrating the feasibility of a non-graphitic anode with sulfide chemistry for potassium-ion batteries. Chem. Commun. 2017, 53, 8272–8275. [Google Scholar] [CrossRef]
- Zhao, L.; Lo, S.H.; He, J.; Li, H.; Biswas, K.; Androulakis, J.; Wu, C.; Hogan, T.P.; Chung, D.Y.; Dravid, V.P.; et al. High performance thermoelectrics from earth-abundant materials: Enhanced Figureure of merit in PbS by second phase nanostructures. J. Am. Chem. Soc. 2011, 113, 20476–20487. [Google Scholar] [CrossRef]
- E, N.M.; Island, J.O.; Mañas-Valero, S.; Pinilla-Cienfuegos, E.; Castellanos-Gomez, A.; Quereda, J.; Rubio-Bollinger, G.; Chirolli, L.; Silva-Guille’n, J.A.; Agraı, N.; et al. Enhanced superconductivity in atomically thin TaS2. Nat. Commun. 2016, 7, 11043. [Google Scholar]
- Hu, Z.; Zhu, Z.; Cheng, F.; Zhang, K.; Wang, J.; Chen, C.; Chen, J. Pyrite FeS2 for high-rate and long-life rechargeable sodium batteries. Energy. Environ. Sci. 2015, 8, 1309–1316. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Liu, Z. Novel WO3/Sb2S3 Heterojunction photocatalyst based on WO3 of different morphologies for enhanced efficiency in photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 9684–9691. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, S.; Zhang, Z.; Lee J, M.; Zapien J, A. Enhanced electrochemical performance of lithium ion batteries using Sb2S3 nanorods wrapped in graphene nanosheets as anode materials. Nanoscale 2018, 10, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.H.; Liu, M. Enhancing sodium ion battery performance by strongly binding nanostructured Sb2S3 on sulfur-doped graphene sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, N.; Jiang, S.; Zhang, Y.; Zhou, M.; Tao, Z.; Archer, L.A.; Chen, J. High-capacity and ultrafast Na-ion storage of a self-supported 3D porous antimony persulfide−graphene foam architecture. Nano Lett. 2017, 17, 3668–3674. [Google Scholar]
- Zhang, W.; Mao, J.; Li, S.; Chen, Z.; Guo, Z. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J. Am. Chem. Soc. 2017, 139, 3316–3319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, J.; Ong, S.; Yao, O.; Shi, X.; Hou, K.; Xu, Z.J.; Guan, L. High-Rate and ultralong cycle-life potassium ion batteries enabled by in situ engineering of yolk–shell FeS2@C structure on graphene matrix. Adv. Energy Mater. 2018, 8, 1802565. [Google Scholar] [CrossRef]
- Mao, M.; Cui, C.; Wu, M.; Zhang, M.; Gao, T.; Fan, X.; Chena, J.; Wang, T.; Ma, J.; Wang, C. Flexible ReS2 nanosheets/N-doped carbon nanofibers-based paper as a universal anode for alkali (Li, Na, K) ion battery. Nano Energy 2018, 45, 346–352. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, P.; Wang, X.; Li, Q.; Dong, Y.; Hua, J.; Zhou, L.; Mai, L. Antimony nanoparticles anchored in three-dimensional carbon network as promising sodium-ion battery anode. J. Power Sources 2016, 304, 340–345. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhang, J.; Pang, W.; Zhang, Q.; Feng, H.; Konstantinov, K.; Guo, Z.; Liu, H. Boosting potassium-ion batteries by few-layered composite anodes prepared via solution-triggered one-step shear exfoliation. Nat. Commun. 2018, 9, 3645. [Google Scholar] [CrossRef]
- Luo, W.; Gaumet, J.-J.; Mai, L. Antimony-based intermetallic compounds for lithium-ion and sodium-ion batteries: Synthesis, construction and application. Rare Met. 2017, 36, 321–338. [Google Scholar] [CrossRef]
- Mai, L.; Tian, X.; Xu, X.; Chang, L.; Xu, L. Nanowire electrodes for electrochemical energy storage devices. Chem. Rev. 2014, 114, 11828–11862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, K.; Xu, W.; Dong, Y.; Xia, R.; Liu, F.; He, L.; Wei, Q.; Yan, M.; Mai, L. Integrated SnO2 nanorod array with polypyrrole coverage for high-rate and long-life lithium batteries. Phys. Chem. Chem. Phys. 2015, 17, 7619–7623. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, K.; Zhitomirsky, I. Anionic dopant–dispersants for synthesis of polypyrrole coated carbon nanotubes and fabrication of supercapacitor electrodes with high active mass loading. J. Mater. Chem. A 2014, 2, 14666–14673. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-up confned synthesis of nanorod-in-nanotube structured Sb@N-C for durable lithium and sodium storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Arumugam, R.; Sellamuthu, K. A novel photocatalytically active mesoporous metal-free PPy grafted MWCNT nanocomposite. J. Colloid Interface Sci. 2018, 514, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xiao, Z.; Miao, L.; Kong, D.; Zheng, X.; Chang, Y.; Zhi, L. Pyrolyzed bacterial cellulose/graphene oxide sandwich interlayer for lithium-sulfur batteries. Rare Met. 2017, 36, 418–424. [Google Scholar] [CrossRef]
- Xie, J.; Liu, L.; Xia, J.; Zhang, Y.; Li, M.; Ouyang, Y.; Nie, S.; Wang, X. Template-Free Synthesis of Sb2S3 Hollow microspheres as anode materials for lithium-ion and sodium-ion batteries. Nano-Micro Lett. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Yang, M.; Wu, J.; Chen, F.; Huang, W.; Han, N.; Ye, H.; Zhao, F.; Li, Y.; Li, Y. Hierarchical VS2 nanosheet assemblies: A universal host material for the reversible storage of alkali metal ions. Adv. Mater. 2017, 29, 1702061. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Prikhodchenko, P.V.; Mason, C.W.; Batabya, K.S.; Gun, J.; Sladkevich, S.; Medvedev, A.G.; Lev, O. High-capacity antimony sulphide nanoparticle decorated graphene composite as anode for sodium-ion batteries. Nat. Commun. 2013, 4, 2922. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, S.; Yin, Y.; Zhu, Y.; Zhang, X.; Yan, J. Green and facile fabrication of MWNTs@Sb2S3@PPy coaxial nanocables for high-performance Na-ion batteries. Part. Part. Syst. Charact. 2016, 33, 493–499. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Zhao, L.; Shen, Y. Flowerlike Sb2S3/PPy microspheres used as anode material for high-performance sodium-ion batteries. Eur. J. Inorg. Chem. 2018, 10, 1224–1228. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Ji, X.; Huang, Z.; Li, S.; Huang, L. Enhanced stability of sodium storage exhibited by carbon coated Sb2S3 hollow spheres. Mater. Chem. Phys. 2017, 203, 185–192. [Google Scholar] [CrossRef]
- Han, C.; Han, K.; Wang, X.; Wang, C.; Li, Q.; Meng, J.; Xu, X.; He, Q.; Luo, W.; Wu, L.; Mai, L. Three-dimensional carbon network confined antimony nanoparticle anodes for high-capacity K-ion batteries. Nanoscale 2018, 10, 6820–6826. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J. Robust self-supported anode by integrating Sb2S3 nanoparticles with, S.; N-codoped graphene to enhance K-storage performance. Sci. China Chem. 2017, 60, 1533–1539. [Google Scholar] [CrossRef]




| SIBs | KIBs | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Electrode Material | Reversible Capacity (mAh g−1) | Cycle Life (mAh g−1) | Rate Performance (mAh g−1) | Reversible Capacity (mAh g−1) | Cycle Life (mAh g−1) | Rate Performance (mAh g−1) | |
| Our work | 940 @ 0.1 A g−1 | 881 @ 0.1 A g−1 after 50 cycles | 940@ 0.1 A g−1 490 @ 1 A g−1 | 700 @ 0.1 A g−1 | 487 @ 0.1 A g−1 after 18 cycles | 690 @ 0.1 A g−1 280 @ 1 A g−1 | |
| CNT@Sb2S3@PPy | 596 @ 0.1 A g−1 | 500 @ 0.1 A g−1 after 80 cycles | 596 @ 0.1 A g−1 400 @ 1 A g−1 | - | - | - | [30] |
| Sb2S3@PPy | 600 @ 0.1 A g−1 | 427 @ 0.1 A g−1 after 50 cycles | 600 @ 0.1 A g−1 500 @ 1 A g−1 | - | - | - | [31] |
| Sb2S3@C | 700 @ 0.2 A g−1 | 650 @ 0.2 A g−1 after 50 cycles | 700 @ 0.2 A g−1 450 @ 0.8 A g−1 | - | - | - | [32] |
| 3D SbNPs@C | - | - | - | 488 @ 0.2 A g−1 | 461 @ 0.2 A g−1 after 15 cycles | 478 @ 0.2 A g−1 288 @ 1 A g−1 | [33] |
| Sb2S3-SNG | - | - | - | 530 @ 0.1 A g−1 | 500 @ 0.1 A g−1 after 100cycles | - | [34] |
| Alkali Metal Ions | Diffusion Coefficient (cm2 s−1) |
|---|---|
| Li+ | 3.16 × 10−10 |
| Na+ | 7 × 10−11 |
| K+ | 1.78 × 10−10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Li, F.; Zhang, Y.; He, L.; Ai, Q.; Luo, W. Sb2S3@PPy Coaxial Nanorods: A Versatile and Robust Host Material for Reversible Storage of Alkali Metal Ions. Nanomaterials 2019, 9, 560. https://doi.org/10.3390/nano9040560
Shi Y, Li F, Zhang Y, He L, Ai Q, Luo W. Sb2S3@PPy Coaxial Nanorods: A Versatile and Robust Host Material for Reversible Storage of Alkali Metal Ions. Nanomaterials. 2019; 9(4):560. https://doi.org/10.3390/nano9040560
Chicago/Turabian StyleShi, Yang, Feng Li, Yi Zhang, Liang He, Qing Ai, and Wen Luo. 2019. "Sb2S3@PPy Coaxial Nanorods: A Versatile and Robust Host Material for Reversible Storage of Alkali Metal Ions" Nanomaterials 9, no. 4: 560. https://doi.org/10.3390/nano9040560





