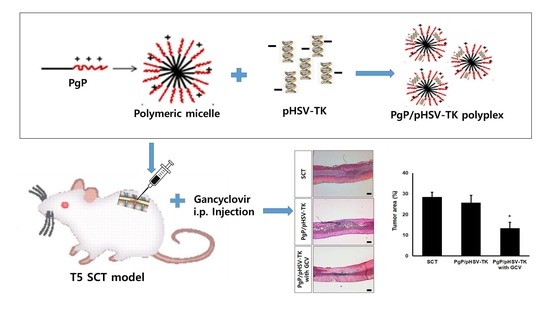
Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor
Abstract
1. Introduction
2. Material and Methods
2.1. Plasmid Amplification and Purification
2.2. Particle Size and Surface Charge of PgP/pDNA Polyplex
2.3. Transfection Efficiency and Cytotoxicity of PgP/pDNA Complex in 10% Serum Condition
2.4. Characterization of PgP/pDNA Polyplexes
2.4.1. Stability of PgP/pDNA Polyplex
2.4.2. Heparin Competition Assay
2.4.3. Stability of PgP/pDNA in Serum
2.5. Long-Term Storage Stability of PgP/pDNA Polyplexes
2.6. Suicide Effects of PgP/pHSV-TK Polyplex and GCV Treatment In Vitro
2.7. Generation of Spinal Cord Tumor Model
2.8. Transfection Efficiency of PgP/pβ-Gal in a Rat Spinal Cord Tumor Model In Vivo
2.9. Suicide Effect of PgP/pHSV-TK Polyplexes with GCV in a Rat Spinal Cord Tumor In Vivo
2.10. Statistical Analysis
3. Results
3.1. Characterization of PgP/pDNA Polyplexes
3.2. Transfection Efficiency and Cytotoxicity of PgP/pDNA Polyplexes in 10% Serum Condition In Vitro
3.3. Stability of PgP/pDNA Polyplex
3.4. Long-Term Storage Stability of PgP/pGFP Polyplexes
3.5. Suicide Effect of PgP/pHSV-TK Polyplex with GSV Treatment In Vitro
3.6. Transfection Efficiency of PgP/pβ-Gal in a Rat Spinal Cord Tumor Model In Vivo
3.7. Suicide Effect of PgP/pHSV-TK Polyplexes with GCV in a Rat Spinal Cord Tumor In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, H.; Avendano, J.; Raza, S.M.; Gokaslan, Z.L.; Jallo, G.I.; Quinones-Hinojosa, A. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: A population-based analysis from 1973 to 2007. Spine 2012, 37, E727–E735. [Google Scholar] [PubMed]
- Mechtler, L.L.; Nandigam, K. Spinal cord tumors: New views and future directions. Neurol. Clin. 2013, 31, 241–268. [Google Scholar] [PubMed]
- Parsa, A.T.; Lee, J.; Parney, I.F.; Weinstein, P.; McCormick, P.C.; Ames, C. Spinal cord and intradural-extraparenchymal spinal tumors: Current best care practices and strategies. J. Neurooncol. 2004, 69, 291–318. [Google Scholar]
- Witham, T.F.; Khavkin, Y.A.; Gallia, G.L.; Wolinsky, J.P.; Gokaslan, Z.L. Surgery insight: Current management of epidural spinal cord compression from metastatic spine disease. Nat. Clin. Pract. Neurol. 2006, 2, 87–94, quiz 116. [Google Scholar]
- Bowers, D.C.; Weprin, B.E. Intramedullary spinal cord tumors. Curr. Treat. Options Neurol. 2003, 5, 207–212. [Google Scholar] [PubMed]
- Legler, J.M.; Ries, L.A.; Smith, M.A.; Warren, J.L.; Heineman, E.F.; Kaplan, R.S.; Linet, M.S. Cancer surveillance series [corrected]: Brain and other central nervous system cancers: Recent trends in incidence and mortality. J. Natl. Cancer Inst. 1999, 91, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Gomez-Manzano, C.; Balague, C.; Yung, W.K.; Curiel, D.T.; Kyritsis, A.P.; Fueyo, J. Gene therapy for gliomas: Molecular targets, adenoviral vectors, and oncolytic adenoviruses. Exp. Cell Res. 1999, 252, 1–12. [Google Scholar] [PubMed]
- Werner-Wasik, M.; Yu, X.; Marks, L.B.; Schultheiss, T.E. Normal-tissue toxicities of thoracic radiation therapy: Esophagus, lung, and spinal cord as organs at risk. Hematol. Oncol. Clin. N. Am. 2004, 18, 131–160, x–xi. [Google Scholar] [CrossRef]
- Balmaceda, C. Chemotherapy for intramedullary spinal cord tumors. J. Neurooncol. 2000, 47, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Sunada, Y. Mechanism of taxane neurotoxicity. Breast Cancer 2004, 11, 82–85. [Google Scholar] [CrossRef]
- Poirier, V.J.; Hershey, A.E.; Burgess, K.E.; Phillips, B.; Turek, M.M.; Forrest, L.J.; Beaver, L.; Vail, D.M. Efficacy and toxicity of paclitaxel (taxol) for the treatment of canine malignant tumors. J. Vet. Intern. Med. 2004, 18, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Pennant, W.A.; An, S.; Gwak, S.J.; Choi, S.; Banh, D.T.; Nguyen, A.B.; Song, H.Y.; Ha, Y.; Park, J.S. Local non-viral gene delivery of apoptin delays the onset of paresis in an experimental model of intramedullary spinal cord tumor. Spinal Cord 2014, 52, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.W.; Kim, K.M.; An, S.S.; Lee, M.; Ha, Y.; Kim, Y.H. Suicide gene therapy using reducible poly (oligo-d-arginine) for the treatment of spinal cord tumors. Biomaterials 2011, 32, 9766–9775. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Maitani, Y. Folate-linked nanoparticle-mediated suicide gene therapy in human prostate cancer and nasopharyngeal cancer with herpes simplex virus thymidine kinase. Cancer Gene Ther. 2005, 12, 796–809. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, L.; Abate-Daga, D.; Rojas, A.; Gonzalez, J.R.; Fillat, C. E-cadherin contributes to the bystander effect of tk/gcv suicide therapy and enhances its antitumoral activity in pancreatic cancer models. Gene Ther. 2011, 18, 73–81. [Google Scholar] [CrossRef][Green Version]
- Pu, K.; Li, S.Y.; Gao, Y.; Ma, L.; Ma, W.; Liu, Y. Bystander effect in suicide gene therapy using immortalized neural stem cells transduced with herpes simplex virus thymidine kinase gene on medulloblastoma regression. Brain Res. 2011, 1369, 245–252. [Google Scholar] [CrossRef]
- Engelmann, C.; Panis, Y.; Bolard, J.; Diquet, B.; Fabre, M.; Nagy, H.; Soubrane, O.; Houssin, D.; Klatzmann, D. Liposomal encapsulation of ganciclovir enhances the efficacy of herpes simplex virus type 1 thymidine kinase suicide gene therapy against hepatic tumors in rats. Hum. Gene Ther. 1999, 10, 1545–1551. [Google Scholar] [CrossRef]
- Kajiwara, E.; Kawano, K.; Hattori, Y.; Fukushima, M.; Hayashi, K.; Maitani, Y. Long-circulating liposome-encapsulated ganciclovir enhances the efficacy of HSV-TK suicide gene therapy. J. Control. Release 2007, 120, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Miura, F.; Moriuchi, S.; Maeda, M.; Sano, A.; Maruno, M.; Tsanaclis, A.M.; Marino, R., Jr.; Glorioso, J.C.; Yoshimine, T. Sustained release of low-dose ganciclovir from a silicone formulation prolonged the survival of rats with gliosarcomas under herpes simplex virus thymidine kinase suicide gene therapy. Gene Ther. 2002, 9, 1653–1658. [Google Scholar] [CrossRef][Green Version]
- Jeon, O.; Yang, H.S.; Lee, T.J.; Kim, B.S. Heparin-conjugated polyethylenimine for gene delivery. J. Control. Release 2008, 132, 236–242. [Google Scholar] [CrossRef]
- Gwak, S.J.; Macks, C.; Jeong, D.U.; Kindy, M.; Lynn, M.; Webb, K.; Lee, J.S. Rhoa knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model. Biomaterials 2017, 121, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Macks, C.; Gwak, S.J.; Lynn, M.; Lee, J.S. Rolipram-loaded polymeric micelle nanoparticle reduces secondary injury after rat compression spinal cord injury. J. Neurotrauma 2018, 35, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.J.; An, S.S.; Yang, M.S.; Joe, E.; Kim, D.H.; Yoon, D.H.; Kim, K.N.; Ha, Y. Effect of combined bevacizumab and temozolomide treatment on intramedullary spinal cord tumor. Spine 2014, 39, E65–E73. [Google Scholar] [CrossRef] [PubMed]
- Gwak, S.J.; Macks, C.; Bae, S.; Cecil, N.; Lee, J.S. Physicochemical stability and transfection efficiency of cationic amphiphilic copolymer/pdna polyplexes for spinal cord injury repair. Sci. Rep. 2017, 7, 11247. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Stealth nanoparticles: High density but sheddable peg is a key for tumor targeting. J. Control. Release 2010, 145, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles escaping res and endosome: Challenges for sirna delivery for cancer therapy. J. Nanomater. 2011, 2011, 11. [Google Scholar] [CrossRef]
- Abdallah, B.; Hassan, A.; Benoist, C.; Goula, D.; Behr, J.P.; Demeneix, B.A. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: Polyethylenimine. Hum. Gene Ther. 1996, 7, 1947–1954. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J. Biomed. Mater. Res. 1999, 45, 268–275. [Google Scholar] [CrossRef]
- Cao, D.; Qin, L.; Huang, H.; Feng, M.; Pan, S.; Chen, J. Transfection activity and the mechanism of pdna-complexes based on the hybrid of low-generation pamam and branched PEI-1.8k. Mol. Biosyst. 2013, 9, 3175–3186. [Google Scholar] [CrossRef]








| N/P ratio | 15 | 30 | 45 | 60 |
|---|---|---|---|---|
| Particle Size (nm) | 141.2 ± 3.8 | 148.5 ± 3.8 | 138.0 ± 3.2 | 145.7 ± 1.5 |
| PDI | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.20 ± 0.01 | 0.17 ± 0.01 |
| Zeta potential (mV) | 34.4 ± 0.2 | 41.3 ± 2.5 | 41.5 ± 0.7 | 41.5 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwak, S.-J.; Lee, J.S. Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor. Nanomaterials 2019, 9, 573. https://doi.org/10.3390/nano9040573
Gwak S-J, Lee JS. Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor. Nanomaterials. 2019; 9(4):573. https://doi.org/10.3390/nano9040573
Chicago/Turabian StyleGwak, So-Jung, and Jeoung Soo Lee. 2019. "Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor" Nanomaterials 9, no. 4: 573. https://doi.org/10.3390/nano9040573
APA StyleGwak, S.-J., & Lee, J. S. (2019). Suicide Gene Therapy By Amphiphilic Copolymer Nanocarrier for Spinal Cord Tumor. Nanomaterials, 9(4), 573. https://doi.org/10.3390/nano9040573