Microstructure and Properties of Self-Assembly Graphene Microcapsules: Effect of the pH Value
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Microcapsules
2.3. Morphology Analysis of Microcapsules
2.4. Shell Thickness and Size Distribution
2.5. Chemical Structure of Shells
2.6. Thermal Properties of Microcapsules
2.7. Nanoindentation Tests
2.8. Compactability of Shells
3. Results and Discussion
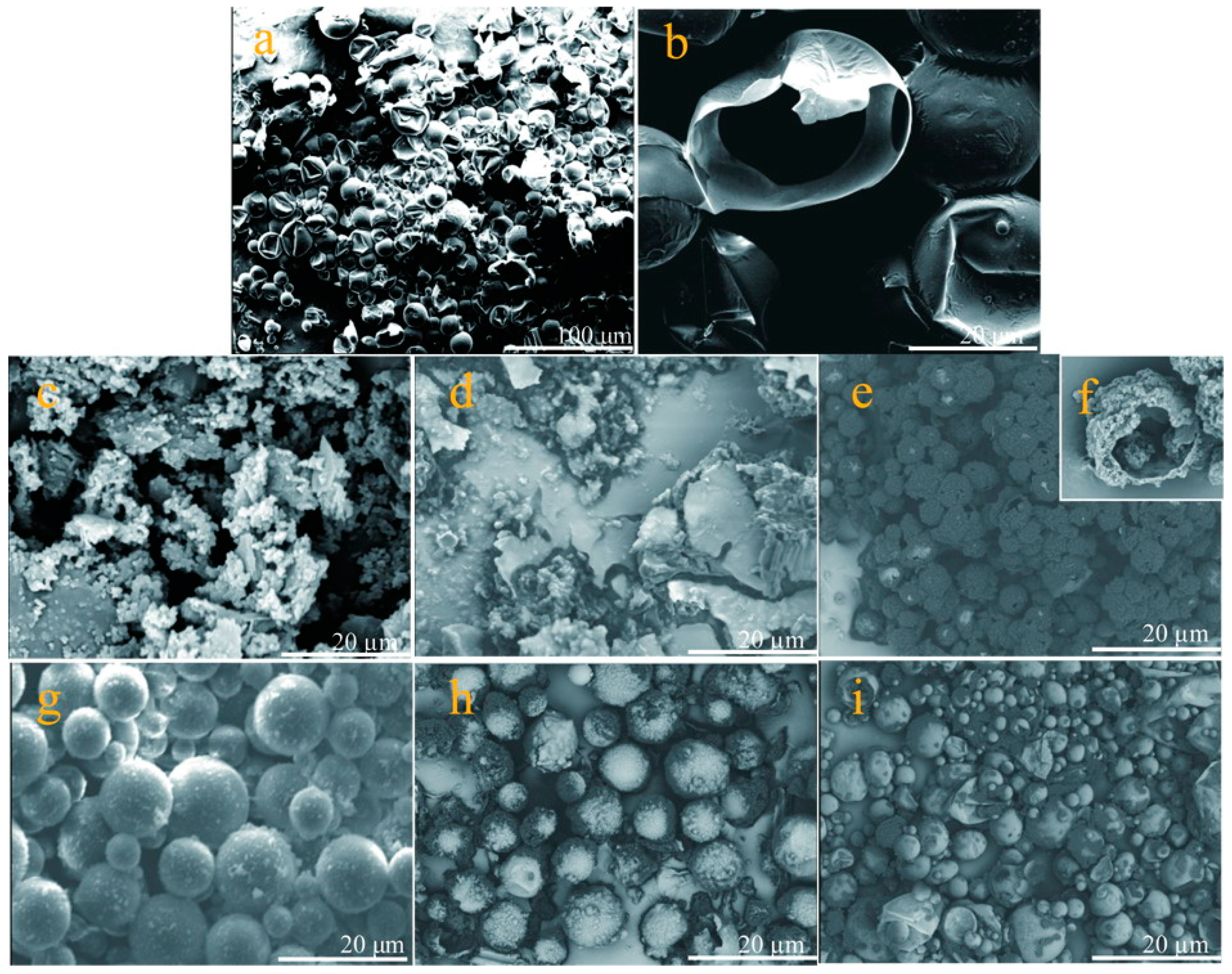
3.1. Morphologies of Microcapsules in Emulsion and Dry States
3.2. Geometry of the Core-Shell Structure
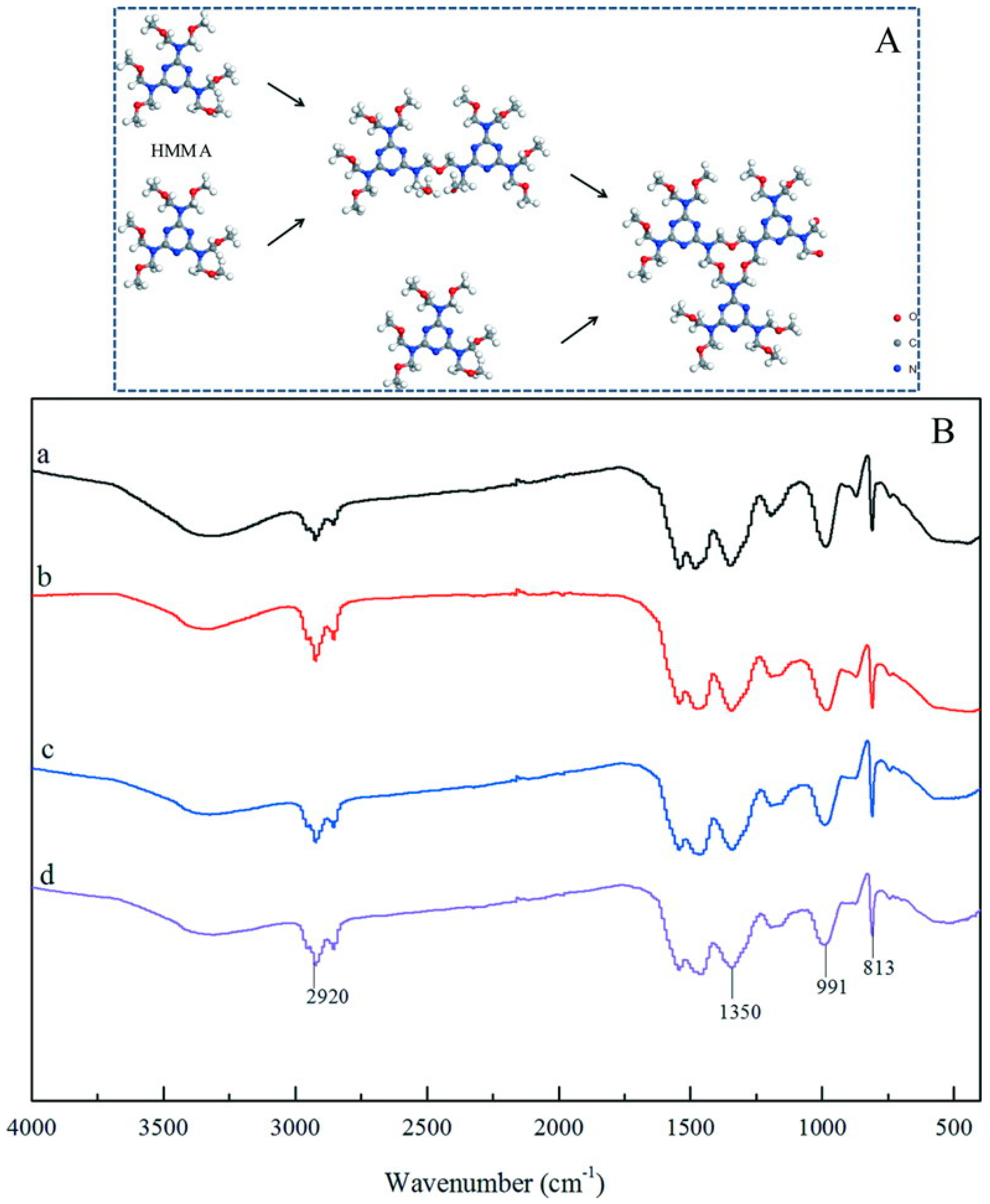
3.3. Chemical Structure of Shells
3.4. Microstructure of Graphene in Shells
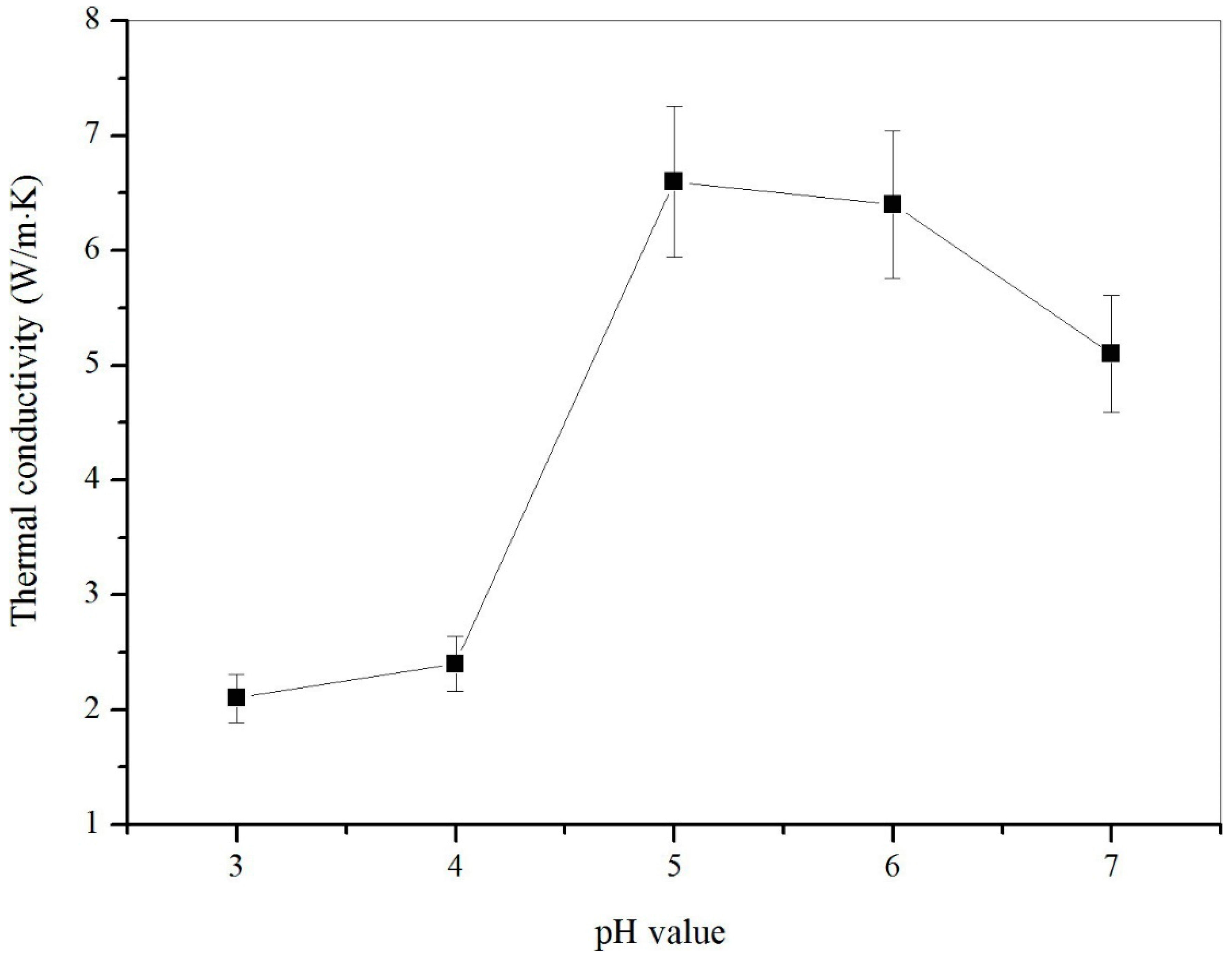
3.5. Thermal Properties of Microcapsules
3.6. Mechanical Property of a Single Microcapsule
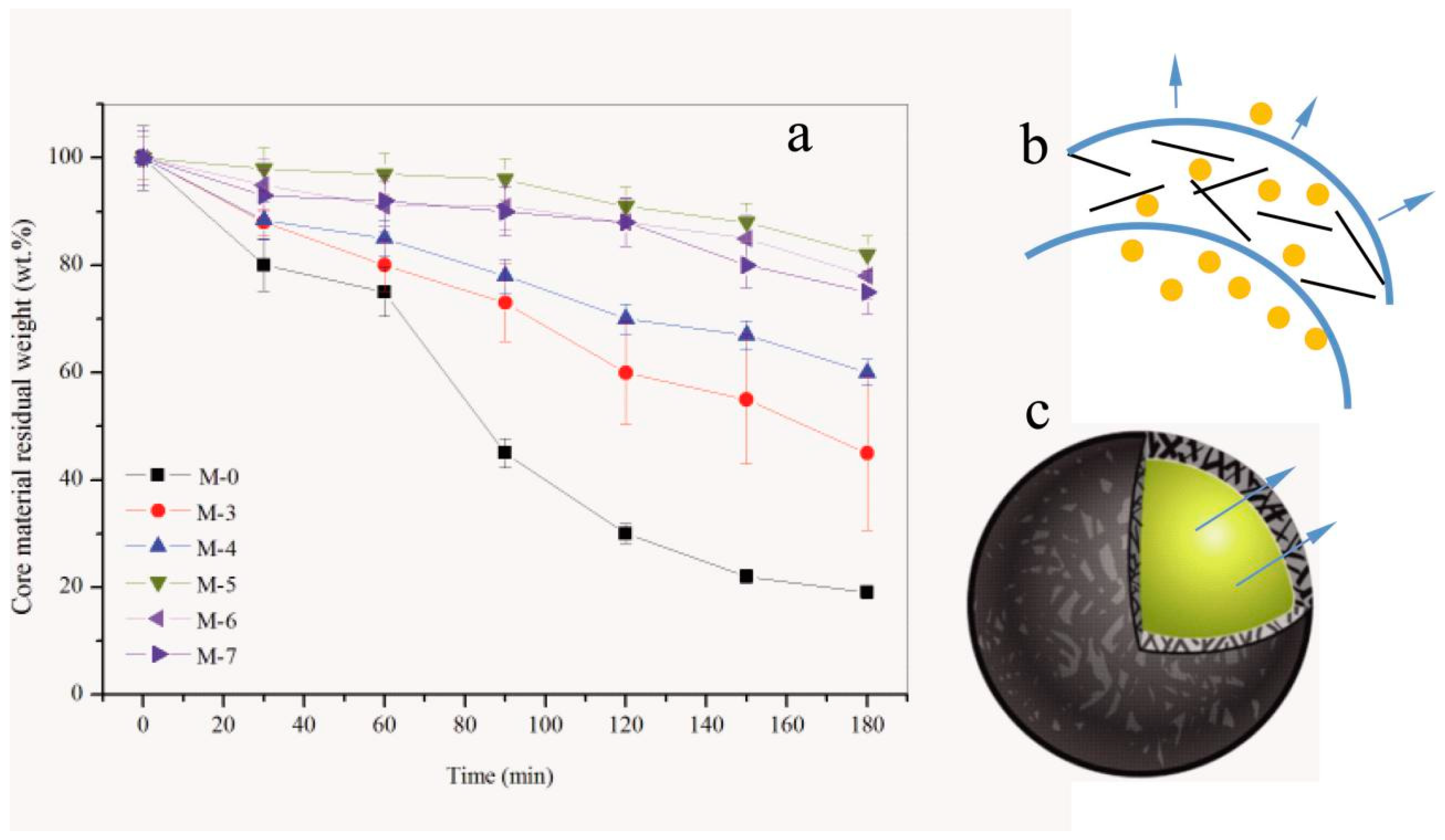
3.7. Compactability of Shells
4. Conclusions
- (1)
- It was proved that several microcapsule samples had been successfully fabricated by the self-assembly process under various pH values in this work. Emulation states and the surface morphologies of microcapsules were observed. The addition amount of graphene was 5% of shell material in the microcapsule. Microcapsules were fabricated under the emulsion speed of 2000 r·min−1 with core/shell weight ratio of 2/1.
- (2)
- The addition of graphene did not change the chemical structure of cross-linked HMMM. The existence of graphene had been proved by the EDS results based on the C element in shells. It was found that the microcapsule sample fabricated under pH = 5 owned the largest graphene content in shells. TEM results were used to investigate the state of graphene in a hybrid microstructure.
- (3)
- This microcapsule sample fabricated under pH=5 sample had the best thermal stability and larger thermal conductivity because of this sample had more graphene in shells. Moreover, a single microcapsule of this sample had the largest yield point tested by nanoindentation.
- (4)
- More graphene decreased the penetrability of core material out of shells. Nanoindentation tests proved this sample had the capability of deforming resistance under pressure coming from the composite structure of graphene/polymer structures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, G.; Brigonli, J.V.A.; Ruhs, P.A.; Studart, A.R. Functional microcapsules with hybrid shells made via sol-gel reaction within double emulsions. Langmuir 2017, 33, 9007–9017. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Wang, X.Y.; Han, S.; Zhang, X.L.; Guo, Y.D.; Wang, Y.Y.; Tan, Y.Q.; Han, N.X.; Li, W. Preparation and physicochemical properties of microcapsules containing phase-change material with graphene/organic hybrid structure shells. J. Mater. Chem. A 2017, 5, 23937–23951. [Google Scholar]
- Nomura, T.; Yoolerd, J.; Sheng, N.; Sakai, H.; Hasegawa, Y.; Haga, M.; Saito, G.; Akiyama, T. Microencapsulation of eutectic and hyper-eutectic Al-Si alloy as phase change materials for high-temperature thermal energy storage. Sol. Energy Mater. Sol. C 2018, 187, 255–262. [Google Scholar]
- Jia, J.; Wang, C.X.; Chen, K.L.; Yin, Y.J. Drug release of yolk/shell microcapsule controlled by pH-responsive yolk swelling. Chem. Eng. J. 2017, 327, 953–961. [Google Scholar]
- Xia, C.M.; Luo, Y.W. The tough microcapsules of acrylic acid-styrene-isoprene-styrene quadrablock copolymer shell via Pickering emulsion technique. J. Appl. Polym. Sci. 2018, 135, 46700. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Leura, A.; Rodriguez, J.F.; Vaselli, O.; Nisi, B.; Higueras, P.L.; Carmona, M. Modelling the mercury removal from polluted waters by using TOMAC microcapsules considering the metal speciation. Chem. Eng. J. 2018, 341, 308–316. [Google Scholar] [CrossRef]
- Zheng, T.; Pilla, S. Encapsulation of hydrophilic payload by PU-PMF capsule: Effect of melamine-formaldehyde pre-polymer content, pH and temperature on capsule morphology. Colloids. Surf. A 2018, 542, 59–67. [Google Scholar] [CrossRef]
- Al Nuumani, R.; Bolognesi, G.; Vladisavljevic, G.T. Microfluidic production of poly(1,6-hexanediol diacrylate)-based polymer microspheres and bifunctional microcapsules with embedded TiO2 nanoparticles. Langmuir 2018, 34, 11822–11831. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, Y.D.; Su, J.F.; Han, S.; Wang, Y.Y.; Tan, Y.Q. Investigating the electrothermal self-healing bituminous composite material using microcapsules containing rejuvenator with graphene/organic hybrid structure shells. Constr. Build. Mater. 2018, 187, 1158–1176. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Hahn, K.; Sanchez, S. Motion control of urea-powered biocompatible hollow microcapsules. ACS Nano 2016, 10, 3597–3605. [Google Scholar]
- Yang, Y.; Zhu, H.K.; Wang, J.; Fang, Q.; Peng, Z.P. Enzymatically disulfide-crosslinked chitosan/hyaluronic acid layer-by-layer self-assembled microcapsules for redox-responsive controlled release of protein. ACS Appl. Mater. Interfaces 2018, 10, 33493–33506. [Google Scholar] [CrossRef] [PubMed]
- Philehvar, S.; Cao, V.D.; Szczotok, A.M.; Carmona, M.; Valentini, L.; Lanzon, M.; Pamies, R.; Kjoniksen, A.L. Physical and mechanical properties of fly ash and slag geopolymer concrete containing different types of micro-encapsulated phase change materials. Constr. Build. Mater. 2018, 173, 28–39. [Google Scholar] [CrossRef]
- Li, K.Z.; Gu, Z.H.; Zhu, X.; Wei, Y.G.; Wang, H. Facile synthesis of Al@Al2O3 microcapsule for high-temperature thermal energy storage. ACS Sustain. Chem. Eng. 2018, 6, 13226–13236. [Google Scholar] [CrossRef]
- Santiago, R.; Lemus, J.; Moreno, D.; Moya, C.; Larriba, M.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J.; Palomar, J. From kinetics to equilibrium control in CO2 capture columns using encapsulated ionic liquids (ENILs). Chem. Eng. J. 2018, 348, 661–668. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Favaro-Trindade, C.S. Physico-chemical properties, stability, and potential food applications of shrimp lipid extract encapsulated by complex coacervation. Food Bioprocess Technol. 2018, 11, 1596–15604. [Google Scholar] [CrossRef]
- Zhang, X.J.; Miao, Z.Y.; Hu, Y.; Yang, X.T.; Tang, Y.Y.; Zhu, D. Programmed microcapsule-type matrix metalloproteinase-2 (MMP-2)-responsive nanosensor for in situ monitoring of intracellular MMP-2. Sens. Actuator B-Chem. 2018, 273, 511–518. [Google Scholar] [CrossRef]
- Kazakova, L.I.; Shabarchina, L.I.; Anastasova, S.; Pavlov, A.M.; Vadgama, P.; Skirtach, A.G.; Sukhorukov, G.B. Chemosensors and biosensors based on polyelectrolyte microcapsules containing fluorescent dyes and enzymes. Anal. Bioanal. Chem. 2013, 405, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.P.; Liu, W.L.; Guo, F.C.; Pei, J.Z.; Zhu, C.Z.; Zhang, W.W. Preparation and characterization of self-healing microcapsules embedding waterborne epoxy resin and curing agent for asphalt materials. Constr. Build. Mater. 2018, 183, 384–394. [Google Scholar] [CrossRef]
- Sun, D.W.; Chong, Y.B.; Chen, K.; Yang, J.L. Chemically and thermally stable isocyanate microcapsules having good self-healing and self- lubricating performances. Chem. Eng. J. 2018, 346, 289–297. [Google Scholar] [CrossRef]
- Guo, M.L.; Li, W.; Han, N.; Wang, J.P.; Su, J.F.; Li, J.J.; Zhang, X.X. Novel dual-component microencapsulated hydrophobic amine and microencapsulated isocyanate used for self-healing anti-corrosion coating. Polymers 2018, 10, 319. [Google Scholar]
- Zhu, K.Y.; Li, X.H.; Su, J.F.; Li, H.; Zhao, Y.H.; Yuan, X.Y. Improvement of anti-icing properties of low surface energy coatings by introducing phase-change microcapsules. Polym. Eng. Sci. 2018, 58, 973–979. [Google Scholar]
- Li, X.M.; Zhang, K.K.; Shi, R.; Ma, X.M.; Tan, L.W.; Ji, Q.; Xia, Y.Z. Enhanced flame-retardant properties of cellulose fibers by incorporation of acid-resistant magnesium-oxide microcapsules. Carbohydr. Polym. 2017, 176, 246–256. [Google Scholar] [PubMed]
- Lan, Y.J.; Chang, S.J.; Li, C.C. Synthesis of conductive microcapsules for fabricating restorable circuits. J. Mater. Chem. A 2017, 5, 25583–25593. [Google Scholar] [CrossRef]
- Kiyoyama, S.; Takayuki, T.; Yoshida, M.; Shiomori, K. Preparation of the highly hygroscopic microcapsules aimed at application of desiccant air conditioner and its hygroscopic properties. Kagaku Kogaku Ronbunshu 2016, 42, 63–67. [Google Scholar]
- Nabavi, S.A.; Vladisavljevic, G.T.; Gu, S.; Manovic, V. Semipermeable Elastic microcapsules for gas capture and sensing. Langmuir 2016, 32, 9826–9835. [Google Scholar]
- Wang, X.F.; Guo, Y.D.; Su, J.F.; Zhang, X.L.; Han, N.X.; Wang, X.Y. Microstructure and thermal reliability of microcapsules containing phase change material with self-assembled graphene/organic nano-hybrid shells. Nanomaterials 2018, 8, 364. [Google Scholar] [CrossRef]
- Li, B.X.; Liu, T.X.; Hu, L.Y.; Wang, Y.F.; Gao, L.N. Fabrication and properties of microencapsulated paraffin@SiO2 phase change composite for thermal energy storage. ACS Sustain. Chem. Eng. 2013, 1, 374–380. [Google Scholar]
- Zhang, Y.M.; Xu, X.; Yu, Q.L.; Liu, Y.H.; Zhang, Y.H.; Chen, L.X.; Liu, Y. Reversing the cytotoxicity of bile acids by supramolecular encapsulation. J. Med. Chem. 2017, 60, 3266–3274. [Google Scholar] [CrossRef]
- Ma, Q.M.; Song, Y.; Kim, J.W.; Choi, H.S.; Shum, H.C. Affinity partitioning-induced self-assembly in aqueous two-phase systems: Templating for polyelectrolyte microcapsules. ACS Macro Lett. 2016, 5, 666–670. [Google Scholar]
- Wang, Y.C.; Li, Y.; Sun, T.M.; Xiong, M.H.; Wu, J.A.; Yang, Y.Y.; Wang, J. Core-shell-corona micelle stabilized by reversible cross-linkage for intracellular drug delivery. Macromol. Rapid Commun. 2010, 31, 1201–1206. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Goyary, D.; Karmakar, S.; Veer, V. Influence of process variables on essential oil microcapsule properties by carbohydrate polymer-protein blends. Carbohydr. Polym. 2013, 93, 691–697. [Google Scholar] [PubMed]
- Yang, P.; Han, S.; Su, J.F.; Wang, Y.Y.; Zhang, X.L.; Han, N.X.; Li, W. Design of self-healing microcapsules containing bituminous rejuvenator with nano-CaCO3/organic composite shell: Mechanical properties, thermal stability, and compactability. Polym. Compos. 2018, 39, e1441–e1451. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Su, J.F.; Schlangen, E.; Han, N.X.; Han, S.; Li, W. Fabrication and characterization of self-healing microcapsules containing bituminous rejuvenator by a nano-inorganic/organic hybrid method. Constr. Build. Mater. 2016, 121, 471–482. [Google Scholar] [CrossRef]
- Su, X.Q.; Wang, G.; Li, W.L.; Bai, J.B.; Wang, H. A simple method for preparing graphene nano-sheets at low temperature. Adv. Powder Technol. 2013, 24, 317–323. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, K.; Lee, B.; Kim, Y.; Hong, B.H. Materials for flexible, stretchable electronics: Graphene and 2D materials. Annu. Rev. Mater. Res. 2015, 45, 63–84. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.H.; Koo, C.M. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Li, J.; Feng, Q.K.; Cui, J.C.; Yuan, Q.Q.; Qiu, H.X.; Gao, S.L.; Yang, J.H. Self-assembled graphene oxide microcapsules in pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar]
- Chen, Z.H.; Wang, J.C.; Yu, F.; Zhang, Z.G.; Gao, X.N. Preparation and properties of graphene oxide modified poly (melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.F.; Khashab, N.M. Hybrid iron oxide-graphene oxide-polysaccharides microcapsule: A micro-matryoshka for on-demand drug release and antitumor therapy in vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef]
- Kaufman, G.; Montejo, K.A.; Michaut, A.; Majewski, P.W.; Osuji, C.O. Photores-ponsive and magnetoresponsive graphene oxide microcapsules fabricated by droplet microfluidics. ACS Appl. Mater. Interfaces 2017, 9, 44192–44198. [Google Scholar] [PubMed]
- Wang, X.F.; Han, R.; Tao, J.; Han, T.L.; Zhu, G.M.; Tang, J.N.; Han, N.X.; Xing, F. Identification of mechanical parameters of urea-formaldehyde microcapsules using finite-element method. Compos. Part B Eng. 2019, 158, 249–258. [Google Scholar]
- Su, J.F.; Schlangen, E.; Qiu, J. Design and construction of microcapsules containing rejuvenator for asphalt. Powder Technol. 2013, 235, 563–571. [Google Scholar] [CrossRef]












| Samples | Stirring Speed (r·min−1) | Core/Shell Ratio | Graphene/Polymer Shell (wt.%) | pH Value |
|---|---|---|---|---|
| M-0 | 2000 | 1/2 | 0 | 4 |
| M-3 | 2000 | 1/2 | 5.0% | 3 |
| M-4 | 2000 | 1/2 | 5.0% | 4 |
| M-5 | 2000 | 1/2 | 5.0% | 5 |
| M-6 | 2000 | 1/2 | 5.0% | 6 |
| M-7 | 2000 | 1/2 | 5.0% | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.-D.; Su, J.-F.; Mu, R.; Wang, X.-Y.; Zhang, X.-L.; Xie, X.-M.; Wang, Y.-Y.; Tan, Y.-Q. Microstructure and Properties of Self-Assembly Graphene Microcapsules: Effect of the pH Value. Nanomaterials 2019, 9, 587. https://doi.org/10.3390/nano9040587
Guo Y-D, Su J-F, Mu R, Wang X-Y, Zhang X-L, Xie X-M, Wang Y-Y, Tan Y-Q. Microstructure and Properties of Self-Assembly Graphene Microcapsules: Effect of the pH Value. Nanomaterials. 2019; 9(4):587. https://doi.org/10.3390/nano9040587
Chicago/Turabian StyleGuo, Yan-Dong, Jun-Feng Su, Ru Mu, Xin-Yu Wang, Xiao-Long Zhang, Xin-Ming Xie, Ying-Yuan Wang, and Yi-Qiu Tan. 2019. "Microstructure and Properties of Self-Assembly Graphene Microcapsules: Effect of the pH Value" Nanomaterials 9, no. 4: 587. https://doi.org/10.3390/nano9040587
APA StyleGuo, Y.-D., Su, J.-F., Mu, R., Wang, X.-Y., Zhang, X.-L., Xie, X.-M., Wang, Y.-Y., & Tan, Y.-Q. (2019). Microstructure and Properties of Self-Assembly Graphene Microcapsules: Effect of the pH Value. Nanomaterials, 9(4), 587. https://doi.org/10.3390/nano9040587




