P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the P4VPPC-co-PDMAPC Copolymer
2.3. Synthesis of the Polymer Films
2.4. Polymer Characterization
2.5. Surface Characterization
2.6. Protein Adsorption Test
2.7. Cell Adhesion Test
3. Results
3.1. Characterization of the P4VPPC-co-PDMAPC Copolymer
3.2. Surface Characterization of Polymer Films
3.3. Antifouling Properties of Polymer Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ladd, J.; Boozer, C.; Yu, Q.; Chen, S.; Homola, J.; Jiang, S. DNA-Directed Protein Immobilization on Mixed Self-Assembled Monolayers via a Streptavidin Bridge. Langmuir 2004, 20, 8090–8095. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Maitz, M.F.; Sperling, C. Current strategies towards hemocompatible coatings. J. Mater. Chem. 2007, 17, 3376–3384. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J.; Chen, L.; Zeng, H. Novel Mussel-Inspired Injectable Self-Healing Hydrogel with Anti-Biofouling Property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef]
- Cho, J.H.; Shanmuganathan, K.; Ellison, C.J. Bioinspired Catecholic Copolymers for Antifouling Surface Coatings. ACS Appl. Mater. Interfaces 2013, 5, 3794–3802. [Google Scholar]
- Dang, Y.; Xing, C.-M.; Quan, M.; Shi, S.-Q.; Zhang, S.-P.; Wang, Y.-B.; Gong, Y.-K. Substrate independent coating formation and anti-biofouling performance improvement of mussel inspired polydopamine. J. Mater. Chem. B 2015, 3, 4181–4190. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar]
- Bernards, M.T.; Cheng, G.; Zhang, Z.; Chen, S.; Jiang, S. Nonfouling Polymer Brushes via Surface-Initiated, Two-Component Atom Transfer Radical Polymerization. Macromolecules 2008, 41, 4216–4219. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A Switchable Biocompatible Polymer Surface with Self-Sterilizing and Nonfouling Capabilities. Angew. Chem. Int. Ed. 2008, 47, 8831–8834. [Google Scholar] [CrossRef] [PubMed]
- Vaisocherova, H.; Sevcu, V.; Adam, P.; Spackova, B.; Hegnerova, K.; de los Santos Pereira, A.; Cesar, R.-E.; Tomáš, R.; Milan, H.; Eduard, B.; Jiří, H. Functionalized ultra-low fouling carboxy- and hydroxy-functional surface platforms: functionalization capacity, biorecognition capability and resistance to fouling from undiluted biological media. Biosens. Bioelectron. 2014, 51, 150–157. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Li, L.; Jiang, S. Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. J. Am. Chem. Soc. 2005, 127, 14473–14478. [Google Scholar] [CrossRef]
- Zheng, J.; Li, L.; Tsao, H.-K.; Sheng, Y.-J.; Chen, S.; Jiang, S. Strong Repulsive Forces between Protein and Oligo (Ethylene Glycol) Self-Assembled Monolayers: A Molecular Simulation Study. Biophys. J. 2005, 89, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Dalsin, J.L.; Lin, L.; Tosatti, S.; Vörös, J.; Textor, M.; Messersmith, P.B. Protein Resistance of Titanium Oxide Surfaces Modified by Biologically Inspired mPEG−DOPA. Langmuir 2005, 21, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Konradi, R.; Acikgoz, C.; Textor, M. Polyoxazolines for Nonfouling Surface Coatings - A Direct Comparison to the Gold Standard PEG. Macromol. Rapid Commun. 2012, 33, 1663–1676. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert Ulrichl, S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Yang, W.; Zhang, Z.; Cao, Z.; Cheng, G.; Piliarik, M.; Homola, J.; Jiang, S. Ultralow Fouling and Functionalizable Surface Chemistry Based on a Zwitterionic Polymer Enabling Sensitive and Specific Protein Detection in Undiluted Blood Plasma. Anal. Chem. 2008, 80, 7894–7901. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “Zero” Protein Adsorption of Poly(carboxybetaine) from Undiluted Blood Serum and Plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.; Manero, O. Thermal and dilute-solution properties of zwitterionic copolymers. J. Polym. Sci. Part B Polym. Phys. 1991, 29, 639–647. [Google Scholar] [CrossRef]
- Shafi, H.Z.; Khan, Z.; Yang, R.; Gleason, K.K. Surface modification of reverse osmosis membranes with zwitterionic coating for improved resistance to fouling. Desalination 2015, 362, 93–103. [Google Scholar] [CrossRef]
- van Zoelen, W.; Asumaa, T.; Ruokolainen, J.; Ikkala, O.; ten Brinke, G. Phase Behavior of Solvent Vapor Annealed Thin Films of PS-b-P4VP(PDP) Supramolecules. Macromolecules 2008, 41, 3199–3208. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Jang, H.; Stocker, R.; Gleason, K.K. Synergistic Prevention of Biofouling in Seawater Desalination by Zwitterionic Surfaces and Low-Level Chlorination. Adv. Mater. 2014, 26, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.-S.; Chen, W.-Y.; Huang, Y.-J.; Cheng, M.-Y.; Yen, Y.-C.; Cheng, C.-C.; Chang, F.-C. Preparation and characterization of high-durability zwitterionic crosslinked proton exchange membranes. J. Membr. Sci. 2010, 362, 29–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, G.; Carr, L.R.; Vaisocherová, H.; Chen, S.; Jiang, S. The hydrolysis of cationic polycarboxybetaine esters to zwitterionic polycarboxybetaines with controlled properties. Biomaterials 2008, 29, 4719–4725. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Jiang, S. Dual-Functional Biomimetic Materials: Nonfouling Poly(carboxybetaine) with Active Functional Groups for Protein Immobilization. Biomacromolecules 2006, 7, 3311–3315. [Google Scholar] [CrossRef]
- Xu, B.; Feng, C.; Hu, J.; Shi, P.; Gu, G.; Wang, L.; Huang, X. Spin-Casting Polymer Brush Films for Stimuli-Responsive and Anti-Fouling Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 6685–6692. [Google Scholar] [CrossRef]
- Sun, X.; Wu, C.; Hu, J.; Huang, X.; Lu, G.; Feng, C. Antifouling Surfaces Based on Fluorine-Containing Asymmetric Polymer Brushes: Effect of Chain Length of Fluorinated Side Chain. Langmuir 2019, 35, 1235–1241. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhu, Q.; Tu, C.; Wang, D.; Wu, J.; Xia, Y.; Tong, G.; He, L.; Zhu, B.; Yan, D.; et al. Protein resistant properties of polymers with different branched architecture on a gold surface. J. Mater. Chem. 2012, 22, 23852–23860. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Ren, K.; Ji, J.; Ji, Y.; Wei, Y. Zwitterionic polycarboxybetaine coating functionalized with REDV peptide to improve selectivity for endothelial cells. J. Biomed. Mater. Res. A 2012, 100, 1387–1397. [Google Scholar]
- Zhang, C.; Yuan, J.; Lu, J.; Hou, Y.; Xiong, W.; Lu, H. From neutral to zwitterionic poly(alpha-amino acid) nonfouling surfaces: Effects of helical conformation and anchoring orientation. Biomaterials 2018, 178, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lü, J.; Hou, Y.; Xiong, W.; Sheng, K.; Lü, H. Investigation on the Linker Length of Synthetic Zwitterionic Polypeptides for Improved Nonfouling Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 17463–17470. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Ruan, H.; Liao, J.; Tan, R.; Pan, J.; Sotto, A.; Gao, C.; Shen, J. Dual Functional Layers Modified Anion Exchange Membranes with Improved Fouling Resistant for Electrodialysis. Adv. Mater. Interfaces 2018, 5, 1800909. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Z.; Tang, Y.; Han, Y.; Lin, Q.; Liu, H.; Chen, H.; Nan, K. Fabrication of nonfouling, bactericidal, and bacteria corpse release multifunctional surface through surface-initiated RAFT polymerization. Int. J. Nanomedicine 2017, 12, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Bernards, M.T.; Cheng, G.; Yu, Q.; Jiang, S. pH responsive properties of non-fouling mixed-charge polymer brushes based on quaternary amine and carboxylic acid monomers. Biomaterials 2010, 31, 2919–2925. [Google Scholar] [CrossRef]


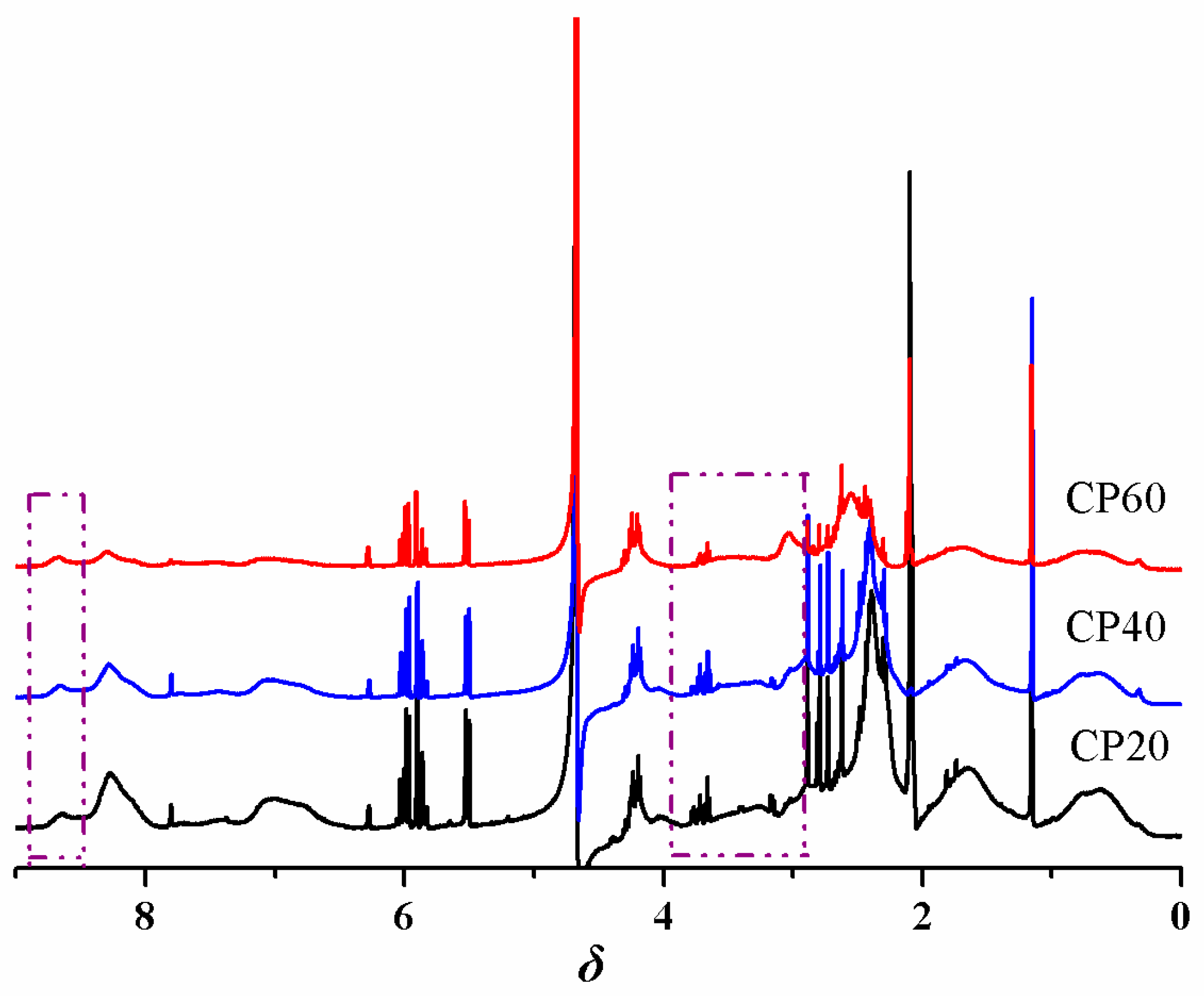
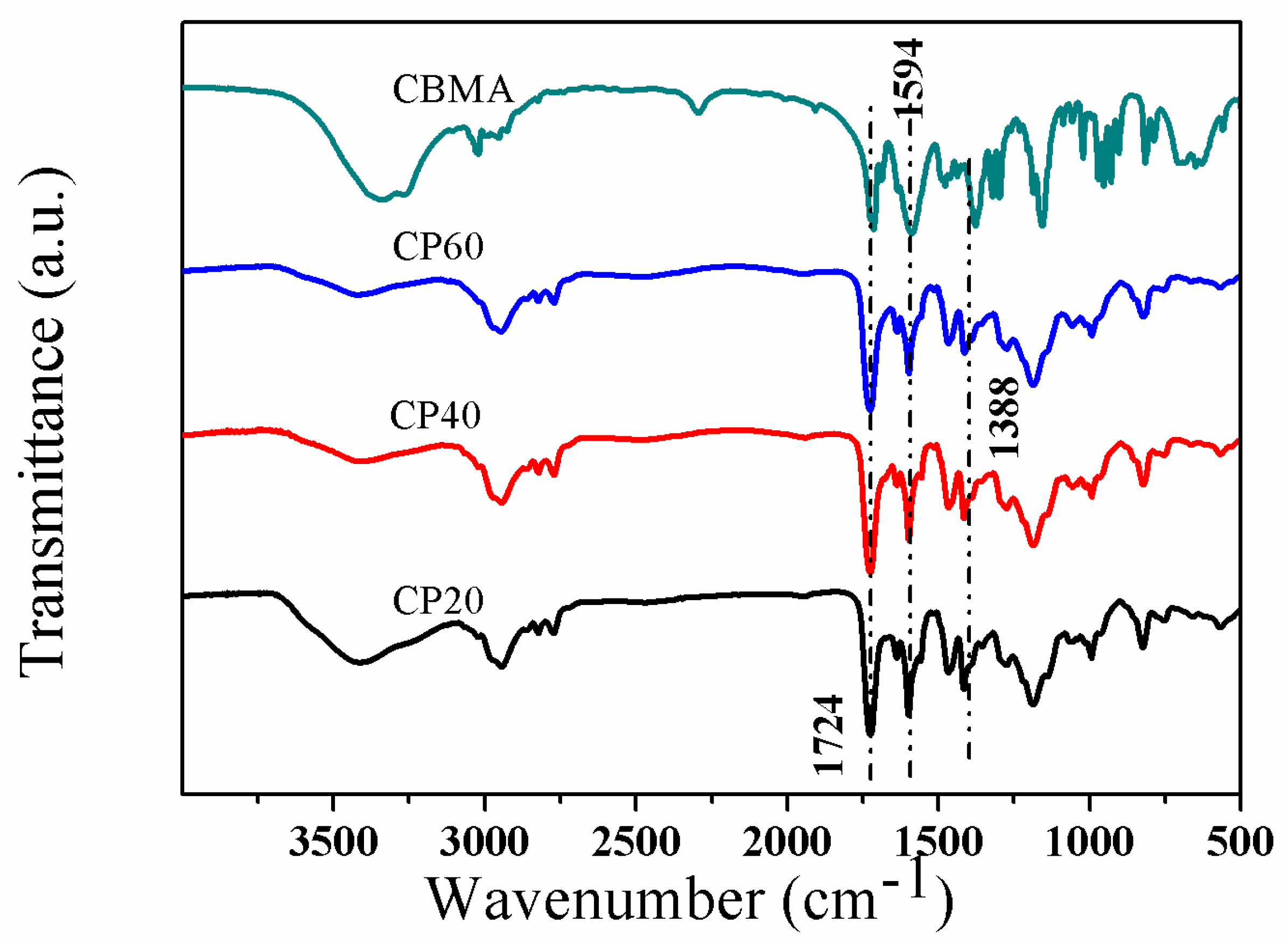

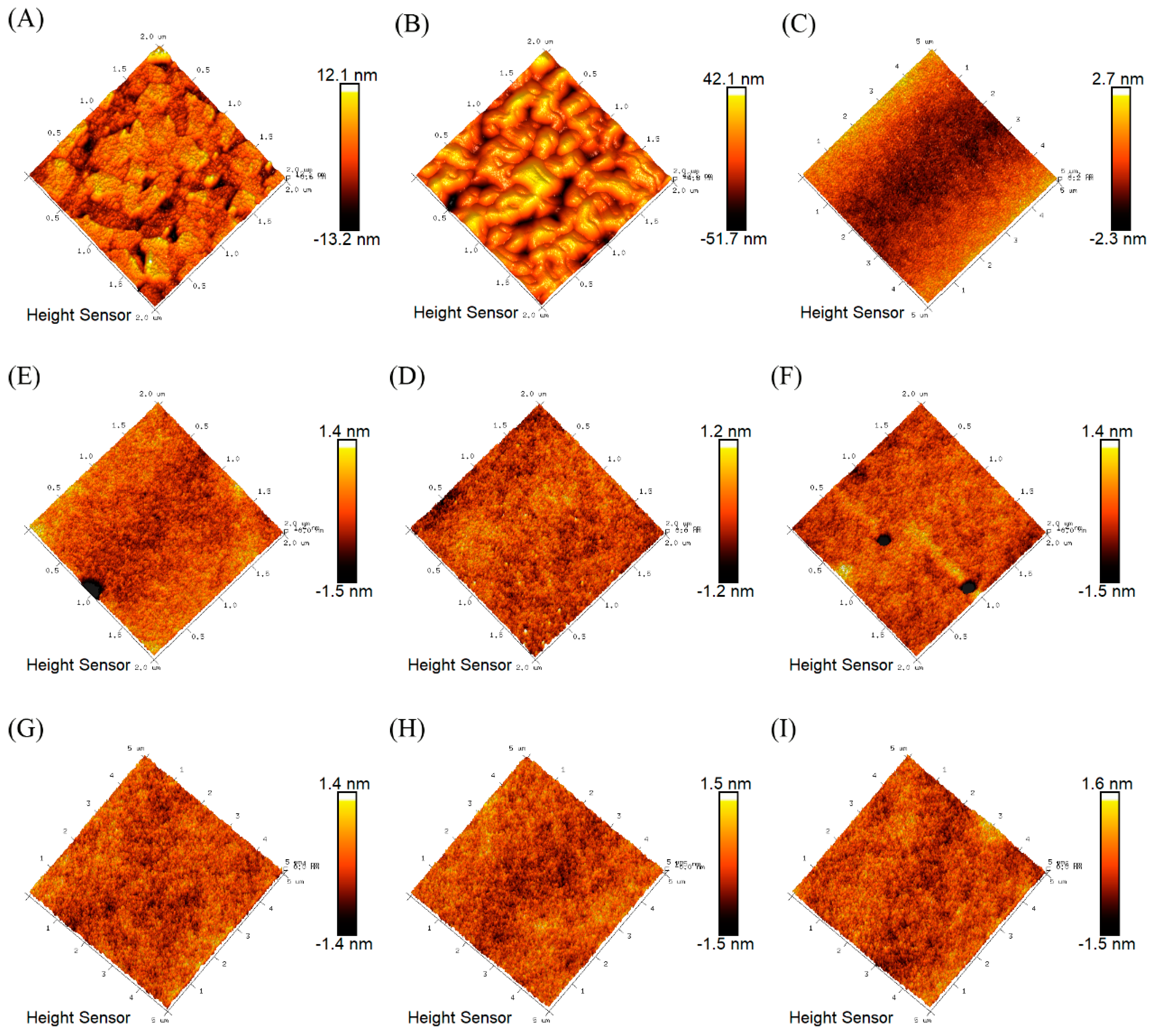



| Entry a | [M]:[AIBN] | Mn b | Mw/Mn b |
|---|---|---|---|
| P4VP | 60:1 | 2540 | 1.11 |
| P1 | 60:1 | 3870 | 1.31 |
| P2 | 180:1 | 4400 | 1.15 |
| P3 | 300:1 | 6600 | 1.24 |
| P4 | 540:1 | 11000 | 1.36 |
| Sample | Si 2p (%) | N 1s (%) | C:O |
|---|---|---|---|
| ITO | 11.57 | 0.86 | 2.36 |
| P4VP | 4.97 | 6.97 | 8.72 |
| P1 | 4.85 | 5.93 | 4.83 |
| P2 | 3.76 | 6.22 | 5.53 |
| P3 | 5.10 | 5.89 | 4.77 |
| P4 | 4.02 | 6.13 | 4.59 |
| CP20 | 5.79 | 5.61 | 4.23 |
| CP40 | 5.47 | 4.57 | 3.94 |
| CP60 | 0 | 4.41 | 3.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhou, Y.; Wang, H.; Hu, J. P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Surfaces. Nanomaterials 2019, 9, 706. https://doi.org/10.3390/nano9050706
Wu C, Zhou Y, Wang H, Hu J. P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Surfaces. Nanomaterials. 2019; 9(5):706. https://doi.org/10.3390/nano9050706
Chicago/Turabian StyleWu, Chaoqun, Yudan Zhou, Haitao Wang, and Jianhua Hu. 2019. "P4VP Modified Zwitterionic Polymer for the Preparation of Antifouling Functionalized Surfaces" Nanomaterials 9, no. 5: 706. https://doi.org/10.3390/nano9050706





