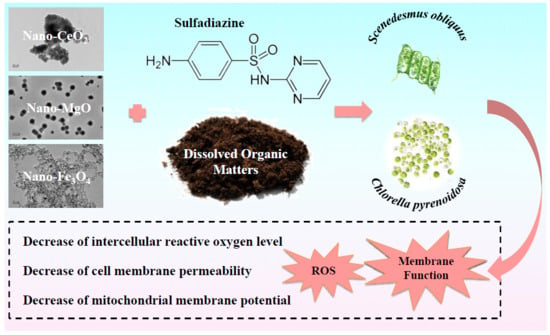
Dissolved Organic Matter Modulates Algal Oxidative Stress and Membrane System Responses to Binary Mixtures of Nano-Metal-Oxides (nCeO2, nMgO and nFe3O4) and Sulfadiazine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials, Test Medium, and Test Species
2.2. Physicochemical Analysis
2.3. Algae Growth, Reactive Oxygen Species, and Membrane Function Assays
3. Results and Discussion
3.1. Physicochemical Characterizations
3.2. Algal Growth Inhibition Toxicity
3.3. Algal Cellular Oxidative Stress Modulation
3.4. Membrane Function Modulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ge, H.L.; Liu, S.S.; Zhu, X.W.; Liu, H.L.; Wang, L.J. Predicting hormetic effects of ionic liquid mixtures on luciferase activity using the concentration addition model. Environ. Sci. Technol. 2011, 45, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Gil, F.; Lacasaña, M. Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223. [Google Scholar] [CrossRef]
- Middepogu, A.; Hou, J.; Gao, X.; Lin, D. Effect and mechanism of TiO2 nanoparticles on the photosynthesis of Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2018, 161, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wu, Y.; Li, X.; Wei, B.; Li, S.; Wang, X. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere 2018, 193, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Vijver, M.G.; Zhai, Y.; Wang, Z.; Peijnenburg, W.J.G.M. Emerging investigator series: The dynamics of particle size distributions need to be accounted for in bioavailability modelling of nanoparticles. Environ. Sci. Nano 2018, 5, 2473–2481. [Google Scholar] [CrossRef]
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Sures, B.; Schmidt, T.C. Ecotoxicity of the two veterinarian antibiotics ceftiofur and cefapirin before and after photo-transformation. Sci. Total Environ. 2018, 619–620, 866–873. [Google Scholar] [CrossRef]
- Iswarya, V.; Sharma, V.; Chandrasekaran, N.; Mukherjee, A. Impact of tetracycline on the toxic effects of titanium dioxide (TiO2) nanoparticles towards the freshwater algal species, Scenedesmus obliquus. Aquat. Toxicol. 2017, 193, 168–177. [Google Scholar] [CrossRef]
- Naasz, S.; Altenburger, R.; Kühnel, D. Environmental mixtures of nanomaterials and chemicals: The Trojan-horse phenomenon and its relevance for ecotoxicity. Sci. Total Environ. 2018, 635, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ji, Y.; Sun, P.; Li, R.; Xiang, F.; Wang, H.; Ruiz-Martinez, J.; Yan, Y. Effects of individual and complex ciprofloxacin, fullerene C60, and ZnO nanoparticles on sludge digestion: Methane production, metabolism, and microbial community. Bioresour. Technol. 2018, 267, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.; Lynch, I.; Van Hoecke, K.; Miermans, C.J.; De Schamphelaere, K.A.; Janssen, C.R.; Dawson, K.A.; Cohen Stuart, M.A.C.; Van De Meent, D. Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 2010, 81, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; Von Der Kammer, F.; Kuhlbusch, T.A.J.; Lead, J.; Nickel, C.; Quik, J.T.K.; Renker, M.; et al. A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134. [Google Scholar] [CrossRef]
- Wang, Z.; Quik, J.T.K.; Song, L.; Van Den Brandhof, E.J.; Wouterse, M.; Peijnenburg, W.J.G.M. Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ. Toxicol. Chem. 2015, 34, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Lin, D.; Zhu, L.; Majumdar, S.; White, J.C.; Gardea-Torresdey, J.L.; Xing, B. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk. Nanotoxicology 2017, 11, 591–612. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M.; Afshinnia, K.; Guo, L. Natural organic matter composition determines the molecular nature of silver nanomaterial-NOM corona. Environ. Sci. Nano 2018, 5, 868–881. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, T.; Guo, X.; Yang, R.; Si, X.; Zhou, J. Humic acid alleviates the ecotoxicity of graphene-family materials on the freshwater microalgae Scenedesmus obliquus. Chemosphere 2018, 197, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Dissolved organic matter and aluminum oxide nanoparticles synergistically cause cellular responses in freshwater microalgae. J. Environ. Sci. Health Part A 2018, 53, 651–658. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Wang, C.; Hou, J.; Yao, Y.; Liu, J.; Lv, B.; Yang, Y.; You, G.; Xu, Y.; et al. Effect of TiO2 and CeO2 nanoparticles on the metabolic activity of surficial sediment microbial communities based on oxygen microelectrodes and high-throughput sequencing. Water Res. 2018, 129, 287–296. [Google Scholar] [CrossRef]
- Ma, B.; Yu, N.; Han, Y.; Gao, M.; Wang, S.; Li, S.; Guo, L.; She, Z.; Zhao, Y.; Jin, C.; et al. Effect of magnesium oxide nanoparticles on microbial diversity and removal performance of sequencing batch reactor. J. Environ. Manag. 2018, 222, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Mashjoor, S.; Yousefzadi, M.; Zolgharnain, H.; Kamrani, E.; Alishahi, M. Organic and inorganic nano-Fe3O4: Alga Ulva flexuosa-based synthesis, antimicrobial effects and acute toxicity to briny water rotifer Brachionus rotundiformis. Environ. Pollut. 2018, 237, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Abdolahpur Monikh, F.; Chupani, L.; Zusková, E.; Peters, R.; Vancová, M.; Vijver, M.G.; Porcal, P.; Peijnenburg, W.J.G.M. Method for extraction and quantification of metal-based nanoparticles in biological media: Number-based biodistribution and bioconcentration. Environ. Sci. Technol. 2019, 53, 946–953. [Google Scholar] [CrossRef]
- Hawthorne, J.; De la Torre Roche, R.; Xing, B.; Newman, L.A.; Ma, X.; Majumdar, S.; Gardea-Torresdey, J.; White, J.C. Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol. 2014, 48, 13102–13109. [Google Scholar] [CrossRef]
- Fajardo, C.; Costa, G.; Nande, M.; Martín, C.; Martín, M.; Sánchez-Fortún, S. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total Environ. 2019, 656, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xue, M.Y.; Luo, F.; Chen, W.C.; Zhu, B.; Wang, G.X. Developmental toxicity of Fe3O4 nanoparticles on cysts and three larval stages of Artemia salina. Environ. Pollut. 2017, 230, 683–691. [Google Scholar] [CrossRef]
- Bayen, S.; Yi, X.; Segovia, E.; Zhou, Z.; Kelly, B.C. Analysis of selected antibiotics in surface freshwater and seawater using direct injection in liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2014, 1338, 38–43. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, L.M.; Ying, G.G.; Liu, C.B.; Zheng, L.Y.; Luo, S.L. Reaction of antibiotic sulfadiazine with manganese dioxide in aqueous phase: Kinetics, pathways and toxicity assessment. J. Environ. Sci. Health Part A 2017, 52, 135–143. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Chen, C.; Du, S.; Dong, Y. Effects of imidazolium chloride ionic liquids and their toxicity to Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2015, 122, 83–90. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Ecotoxicological effects of enrofloxacin and its removal by monoculture of microalgal species and their consortium. Environ. Pollut. 2017, 226, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xin, H.; Yang, F.; Long, X. A historical review and bibliometric analysis of nanoparticles toxicity on algae. J. Nanopart. Res. 2018, 20, 92. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y.; Wang, Z.; Ren, W.; Wei, Y.; Cao, X.; Xing, B. Toxicity of GO to freshwater algae in the presence of Al2O3 particles with different morphologies: Importance of heteroaggregation. Environ. Sci. Technol. 2018, 52, 13448–13456. [Google Scholar] [CrossRef]
- OECD. Freshwater Alga and Cyanobacteria, Growth Inhibition Test., Nr. 201, OECD Guidelines for the Testing of Chemicals; Organization for Economic Cooperation and Development (OECD): Paris, France, 2011; Available online: http://www.oecd.org (accessed on 25 March 2019).
- Bhuvaneshwari, M.; Iswarya, V.; Archanaa, S.; Madhu, G.M.; Kumar, G.K.S.; Nagarajan, R.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat. Toxicol. 2015, 162, 29–38. [Google Scholar] [CrossRef]
- Iswarya, V.; Bhuvaneshwari, M.; Alex, S.A.; Iyer, S.; Chaudhuri, G.; Chandrasekaran, P.T.; Bhalerao, G.M.; Chakravarty, S.; Raichur, A.M.; Chandrasekaran, N.; et al. Combined toxicity of two crystalline phases (anatase and rutile) of Titania nanoparticles towards freshwater microalgae: Chlorella sp. Aquat. Toxicol. 2015, 161, 154–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Chen, M.; Fang, H.; Wang, D. Co-exposure of freshwater microalgae to tetrabromobisphenol A and sulfadiazine: Oxidative stress biomarker responses and joint toxicity prediction. Bull. Environ. Contam. Toxicol. 2017, 99, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, X.; Han, X.; Tang, Z.; Song, F.; Zhang, S.; Zhu, Y.; Guo, W.; He, Z.; Guo, Q.; et al. Colloidal stability of Fe3O4 magnetic nanoparticles differentially impacted by dissolved organic matter and cations in synthetic and naturally-occurred environmental waters. Environ. Pollut. 2018, 241, 912–921. [Google Scholar] [CrossRef]
- Baalousha, M.; Manciulea, A.; Cumberland, S.; Kendall, K.; Lead, J.R. Aggregation and surface properties of iron oxide nanoparticles: Influence of pH and natural organic matter. Environ. Toxicol. Chem. 2008, 27, 1875–1882. [Google Scholar] [CrossRef]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Odnevall Wallinder, I. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanopart. Res. 2016, 18, 285. [Google Scholar] [CrossRef]
- Shin, Y.J.; Lee, W.M.; Kwak, J.I.; An, Y.J. Dissolution of zinc oxide nanoparticles in exposure media of algae, daphnia, and fish embryos for nanotoxicological testing. Chem. Ecol. 2018, 34, 229–240. [Google Scholar] [CrossRef]
- Sendra, M.; Moreno-Garrido, I.; Blasco, J.; Araújo, C.V.M. Effect of erythromycin and modulating effect of CeO2 NPs on the toxicity exerted by the antibiotic on the microalgae Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Environ. Pollut. 2018, 242, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Lei, C.; Zhang, L.; Yang, K.; Zhu, L.; Lin, D. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging. Environ. Pollut. 2016, 218, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of antibacterial activity of MgO: Non-ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Pavelescu, L.A. On reactive oxygen species measurement in living systems. J. Med. Life 2015, 8, 38–42. [Google Scholar] [PubMed]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, S.; Quan, X.; Jin, Y.H. Toxic effect of serial perfluorosulfonic and perfluorocarboxylic acids on the membrane system of a freshwater alga measured by flow cytometry. Environ. Toxicol. Chem. 2008, 27, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Quigg, A.; Chin, W.C.; Chen, C.S.; Zhang, S.; Jiang, Y.; Miao, A.J.; Schwehr, K.A.; Xu, C.; Santschi, P.H. Direct and indirect toxic effects of engineered nanoparticles on algae: Role of natural organic matter. ACS Sustain. Chem. Eng. 2013, 1, 686–702. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhao, J.; Xing, B. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]






| nMeO | Fraction (%) | |
|---|---|---|
| 0 min | 30 min | |
| nCeO2 | 0.02 ± 0.00 | 0.03 ± 0.01 |
| nMgO | 0.69 ± 0.04 | 0.81 ± 0.16 |
| nFe3O4 | 38.41 ± 3.02 | 34.34 ± 2.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Ye, N.; Wang, S.; Meng, Y.; Fang, H.; Wang, Z.; Wang, D.-G. Dissolved Organic Matter Modulates Algal Oxidative Stress and Membrane System Responses to Binary Mixtures of Nano-Metal-Oxides (nCeO2, nMgO and nFe3O4) and Sulfadiazine. Nanomaterials 2019, 9, 712. https://doi.org/10.3390/nano9050712
Zhang F, Ye N, Wang S, Meng Y, Fang H, Wang Z, Wang D-G. Dissolved Organic Matter Modulates Algal Oxidative Stress and Membrane System Responses to Binary Mixtures of Nano-Metal-Oxides (nCeO2, nMgO and nFe3O4) and Sulfadiazine. Nanomaterials. 2019; 9(5):712. https://doi.org/10.3390/nano9050712
Chicago/Turabian StyleZhang, Fan, Nan Ye, Se Wang, Yue Meng, Hao Fang, Zhuang Wang, and De-Gao Wang. 2019. "Dissolved Organic Matter Modulates Algal Oxidative Stress and Membrane System Responses to Binary Mixtures of Nano-Metal-Oxides (nCeO2, nMgO and nFe3O4) and Sulfadiazine" Nanomaterials 9, no. 5: 712. https://doi.org/10.3390/nano9050712