Dual Substitution and Spark Plasma Sintering to Improve Ionic Conductivity of Garnet Li7La3Zr2O12
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis and Free Sintering
2.2. SPS Sintering
2.3. Characterization
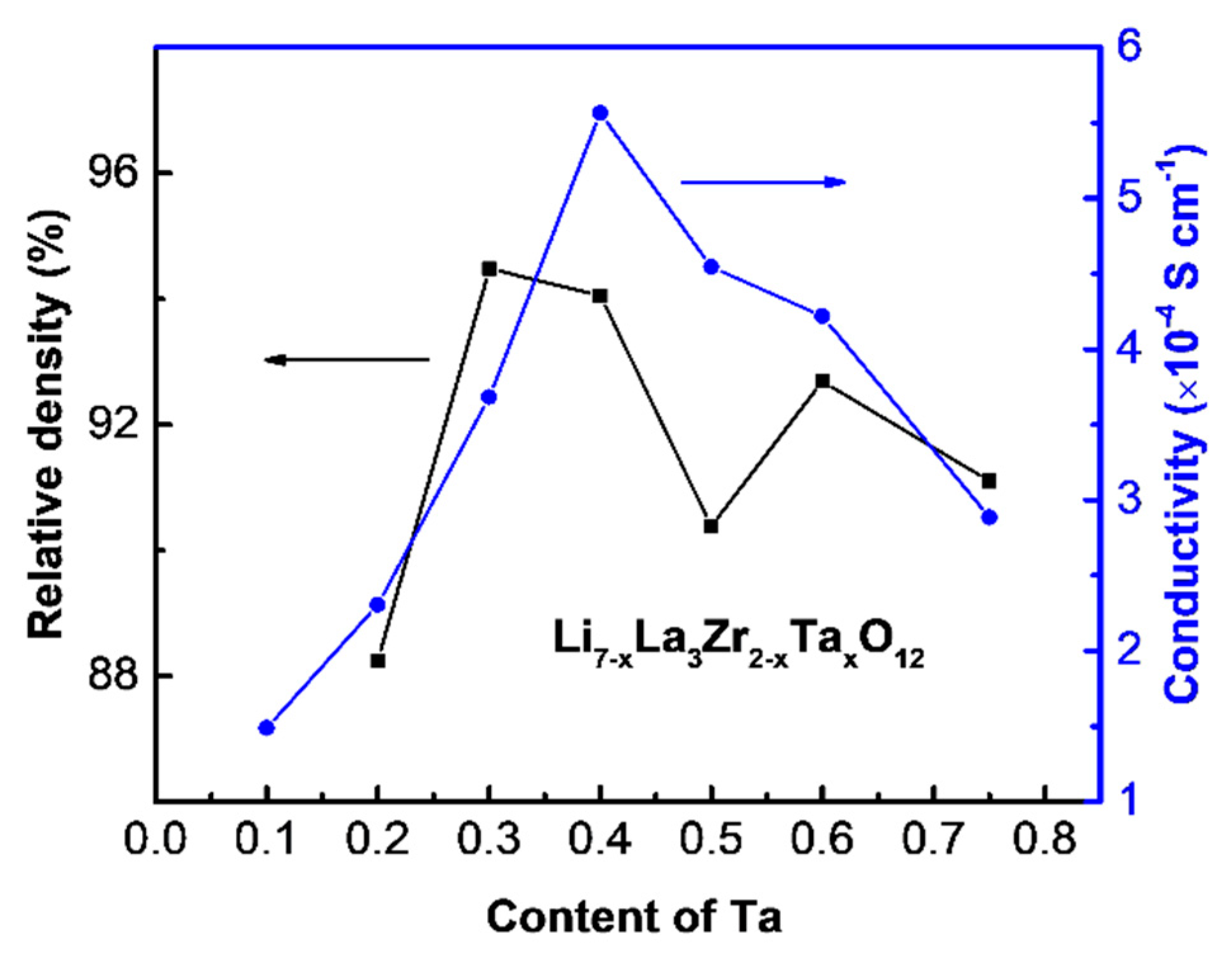
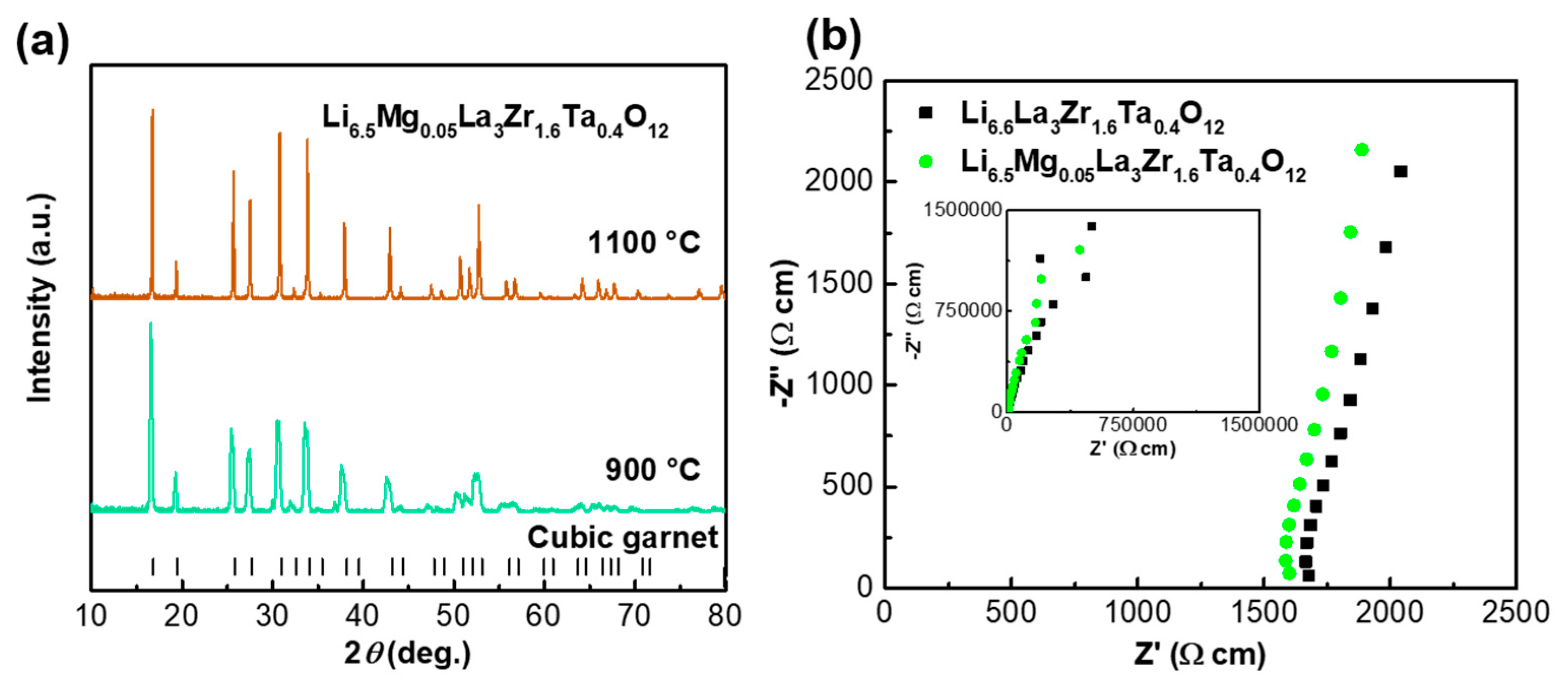
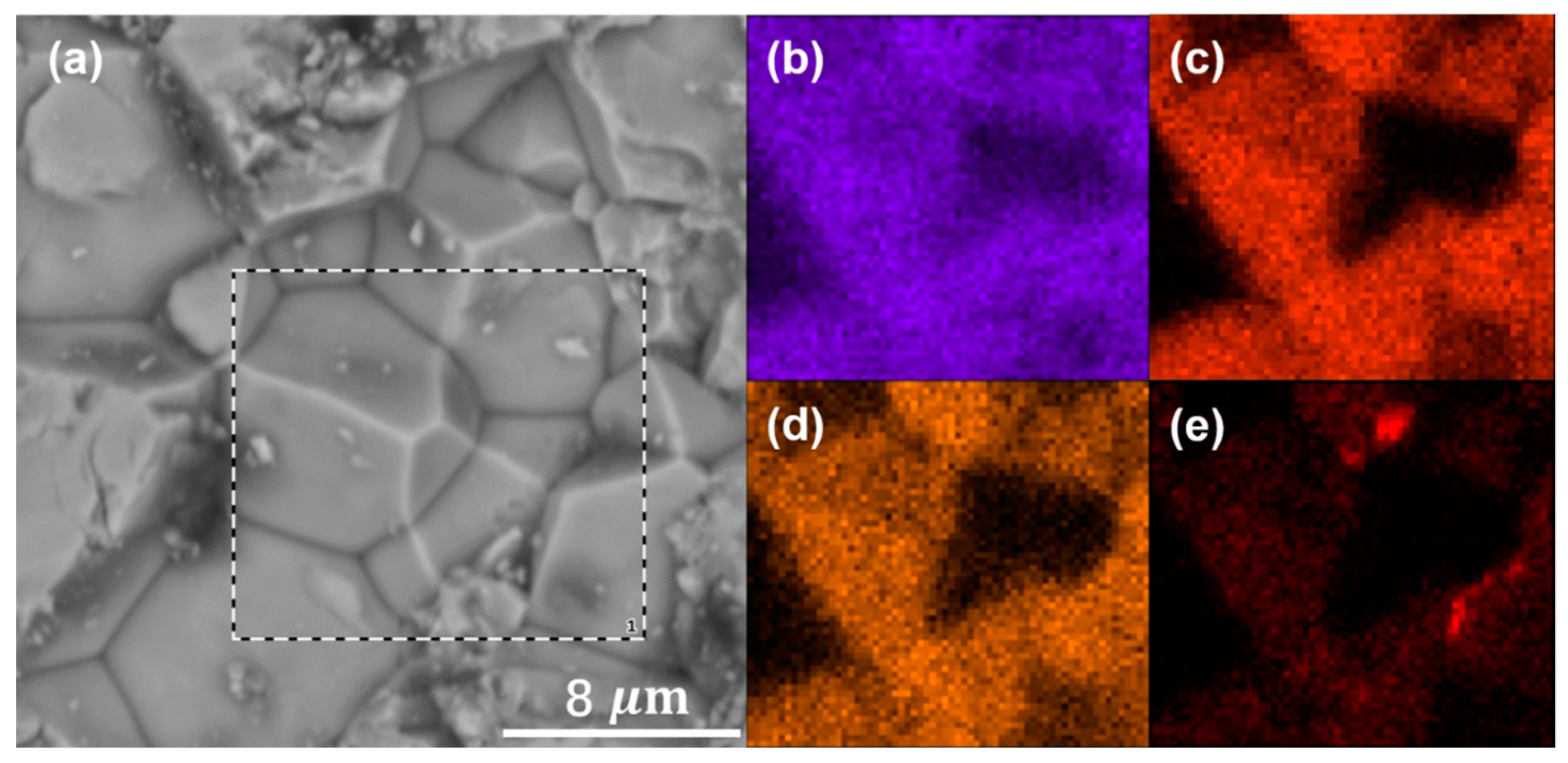
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.S.; Jiang, L.H.; Yu, Y.; Sun, J.H. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, X.R.; Li, H.X.; Wu, H.B. Recent Progress of Hybrid Solid-State Electrolytes for Lithium Batteries. Chem.-Eur. J. 2018, 24, 18293–18306. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Ma, Q.L.; Dellen, C.; Lobe, S.; Vondahlen, F.; Windmuller, A.; Gruner, D.; Zheng, H.; Uhlenbruck, S.; Finsterbusch, M.; et al. A garnet structure-based all-solid-state Li battery without interface modification: Resolving incompatibility issues on positive electrodes. Sustain. Energ. Fuels 2019, 3, 280–291. [Google Scholar] [CrossRef]
- Yeandel, S.R.; Chapman, B.J.; Slater, P.R.; Goddard, P. Structure and lithium-ion dynamics in fluoride-doped cubic Li7La3Zr2O12 (LLZO) garnet for Li solid-state battery applications. J. Phys. Chem. C 2018, 122, 27811–27819. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Cussen, E.J.; Corr, S.A. Enhancement of the lithium ion conductivity of Ta-doped Li7La3Zr2O12 by incorporation of calcium. Dalton Trans. 2017, 46, 9415–9419. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Ratchford, J.; Rangasamy, E.; Sakamoto, J.; Allen, J.L. Synthesis and high Li-ion conductivity of Ga-stabilized cubic Li7La3Zr2O12. Mater. Chem. Phys. 2012, 134, 571–575. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, D.B.; Chen, Z.P.; Sun, Y.Y.; Liu, Y.Q. Low-temperature fabrication for transparency Mg doping Li7La3Zr2O12 solid state electrolyte. J. Mater. Sci.-Mater. Electron. 2018, 29, 1523–1529. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, C.; Rong, T.; Qiang, S.; Zhang, L. Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. J. Power Sources 2014, 268, 960–964. [Google Scholar] [CrossRef]
- Yamada, H.; Ito, T.; Basappa, R.H. Sintering mechanisms of high-performance garnet-type solid electrolyte densified by spark plasma sintering. Electrochim. Acta 2016, 222, 648–656. [Google Scholar] [CrossRef]
- Baek, S.W.; Lee, J.M.; Kim, T.Y.; Song, M.S.; Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2014, 249, 197–206. [Google Scholar] [CrossRef]
- Benavente, R.; Pruna, A.; Borrell, A.; Salvador, M.D.; Pullini, D.; Penaranda-Foix, F.; Busquets, D. Fast route to obtain Al2O3-based nanocomposites employing graphene oxide: Synthesis and sintering. Mater. Res. Bull. 2015, 64, 245–251. [Google Scholar] [CrossRef]
- Gordon, Z.D.; Yang, T.; Morgado, G.B.G.; Chan, C.K. Preparation of nano- and microstructured garnet Li7La3Zr2O12 solid electrolytes for Li-ion batteries via cellulose templating. ACS Sustain. Chem. Eng. 2016, 4, 6391–6398. [Google Scholar] [CrossRef]
- Liu, K.; Ma, J.T.; Wang, C.A. Excess lithium salt functions more than compensating for lithium loss when synthesizing Li6.5La3Ta0.5Zr1.5O12 in alumina crucible. J. Power Sources 2014, 260, 109–114. [Google Scholar] [CrossRef]
- Li, Y.Q.; Cao, Y.; Guo, X.X. Influence of lithium oxide additives on densification and ionic conductivity of garnet-type Li6.75La3Zr1.75Ta0.25O12 solid electrolytes. Solid State Ionics 2013, 253, 76–80. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zhang, L.; Zhang, J.; Aggarwal, S. Removal of arsenic(III) from aqueous solution using metal organic framework-graphene oxide nanocomposite. Nanomaterials 2018, 8, 1062. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Bai, Y.; Wen, Y.; Zhao, N.; Zhang, X.; Zhang, Y.; Li, Q.; Wei, L. The preparation and properties of nanocomposite from bio-based polyurethane and graphene oxide for gas separation. Nanomaterials 2019, 9, 15. [Google Scholar] [CrossRef]
- Logeat, A.; Koohler, T.; Eisele, U.; Stiaszny, B.; Harzer, A.; Tovar, M.; Senyshyn, A.; Ehrenberg, H.; Kozinsky, B. From order to disorder: The structure of lithium-conducting garnets Li7-xLa3TaxZr2-xO12 (x=0-2). Solid State Ionics 2012, 206, 33–38. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lai, W. High ionic conductivity lithium garnet oxides of Li7-xLa3Zr2-xTaxO12 compositions. Electrochem. Solid State Lett. 2012, 15, A68–A71. [Google Scholar] [CrossRef]
- Li, Y.T.; Xu, B.Y.; Xu, H.H.; Duan, H.N.; Lu, X.J.; Xin, S.; Zhou, W.D.; Xue, L.G.; Fu, G.T.; Manthiram, A.; et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries. Angew. Chem.-Int. Ed. 2017, 56, 753–756. [Google Scholar] [CrossRef]
- Schleutker, M.; Bahner, J.; Tsai, C.L.; Stolten, D.; Korte, C. On the interfacial charge transfer between solid and liquid Li+ electrolytes. Phys. Chem. Chem. Phys. 2017, 19, 26596–26605. [Google Scholar] [CrossRef]


| Compound | Lattice Parameter (Å) | Ref. | Theoretical Density (g cm−3) | Relative Density (%) |
|---|---|---|---|---|
| Li6.25La3Zr1.25Ta0.75O12 | 12.91553(6) | [20] | 5.561 | 91.10 |
| Li6.4La3Zr1.4Ta0.6O12 | 12.923 | [21] | 5.551 | 92.69 |
| Li6.5La3Zr1.5Ta0.5O12 | 12.9340 | [23] | 5.410 | 90.24 |
| Li6.6La3Zr1.6Ta0.4O12 | 12.939 | [24] | 5.352 | 94.05 |
| Li6.7La3Zr1.7Ta0.3O12 | 12.9721 | [21] | 5.261 | 94.48 |
| Li6.8La3Zr1.8Ta0.2O12 | 12.9780 | [22] | 5.203 | 88.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Xu, C.; Wu, Y.; Tang, W.; Song, S.; Yao, J.; Huang, Z.; Wen, Z.; Lu, L.; Hu, N. Dual Substitution and Spark Plasma Sintering to Improve Ionic Conductivity of Garnet Li7La3Zr2O12. Nanomaterials 2019, 9, 721. https://doi.org/10.3390/nano9050721
Dong Z, Xu C, Wu Y, Tang W, Song S, Yao J, Huang Z, Wen Z, Lu L, Hu N. Dual Substitution and Spark Plasma Sintering to Improve Ionic Conductivity of Garnet Li7La3Zr2O12. Nanomaterials. 2019; 9(5):721. https://doi.org/10.3390/nano9050721
Chicago/Turabian StyleDong, Zhencai, Chao Xu, Yongmin Wu, Weiping Tang, Shufeng Song, Jianyao Yao, Zhengyong Huang, Zhaoyin Wen, Li Lu, and Ning Hu. 2019. "Dual Substitution and Spark Plasma Sintering to Improve Ionic Conductivity of Garnet Li7La3Zr2O12" Nanomaterials 9, no. 5: 721. https://doi.org/10.3390/nano9050721
APA StyleDong, Z., Xu, C., Wu, Y., Tang, W., Song, S., Yao, J., Huang, Z., Wen, Z., Lu, L., & Hu, N. (2019). Dual Substitution and Spark Plasma Sintering to Improve Ionic Conductivity of Garnet Li7La3Zr2O12. Nanomaterials, 9(5), 721. https://doi.org/10.3390/nano9050721






