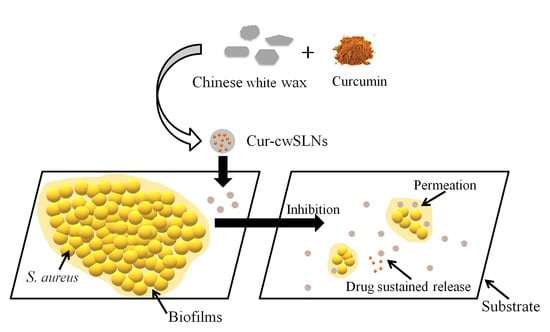
Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Empty Chinese White Wax SLNs (cwSLNs)
2.2.1. Formula Optimization of the cwSLNs
- (a)
- HT = (20, 30, and 40 min), while ST = 4 min, SC = 1% (v/v), and LC = 1% (w/v);
- (b)
- ST = (6, 8, and 10 min), while HT = 40 min, SC = 3% (v/v), and LC = 1% (w/v);
- (c)
- SC = (1%, 3%, and 6%, v/v), while HT = 40 min, ST = 10 min, and LC = 1% (w/v);
- (d)
- LC = (0.5%, 1%, and 2%, w/v), while HT = 40 min, ST = 10 min, and SC = 3% (v/v).
2.2.2. Preparation of Curcumin Loaded Chinese White Wax SLNs (Cur-cwSLNs)
2.3. Characterization of Chinese White Wax-Based SLNs
2.3.1. Determination of Particle Size and Polydispersity
2.3.2. Zeta Potential Measurement
2.3.3. Entrapment Efficiency
2.3.4. ABTS Assay
2.3.5. Transmission Electron Microscopy (TEM)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. In Vitro Drug Release and Cytotoxicity
2.4. In Vitro Anti-Biofilm Study
2.4.1. Determination of the Minimum Inhibitory Concentration (MIC)
2.4.2. Effects of Cur-cwSLNs on Reducing the Formation of S. aureus Biofilm
2.4.3. SEM Observation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Blank cwSLNs
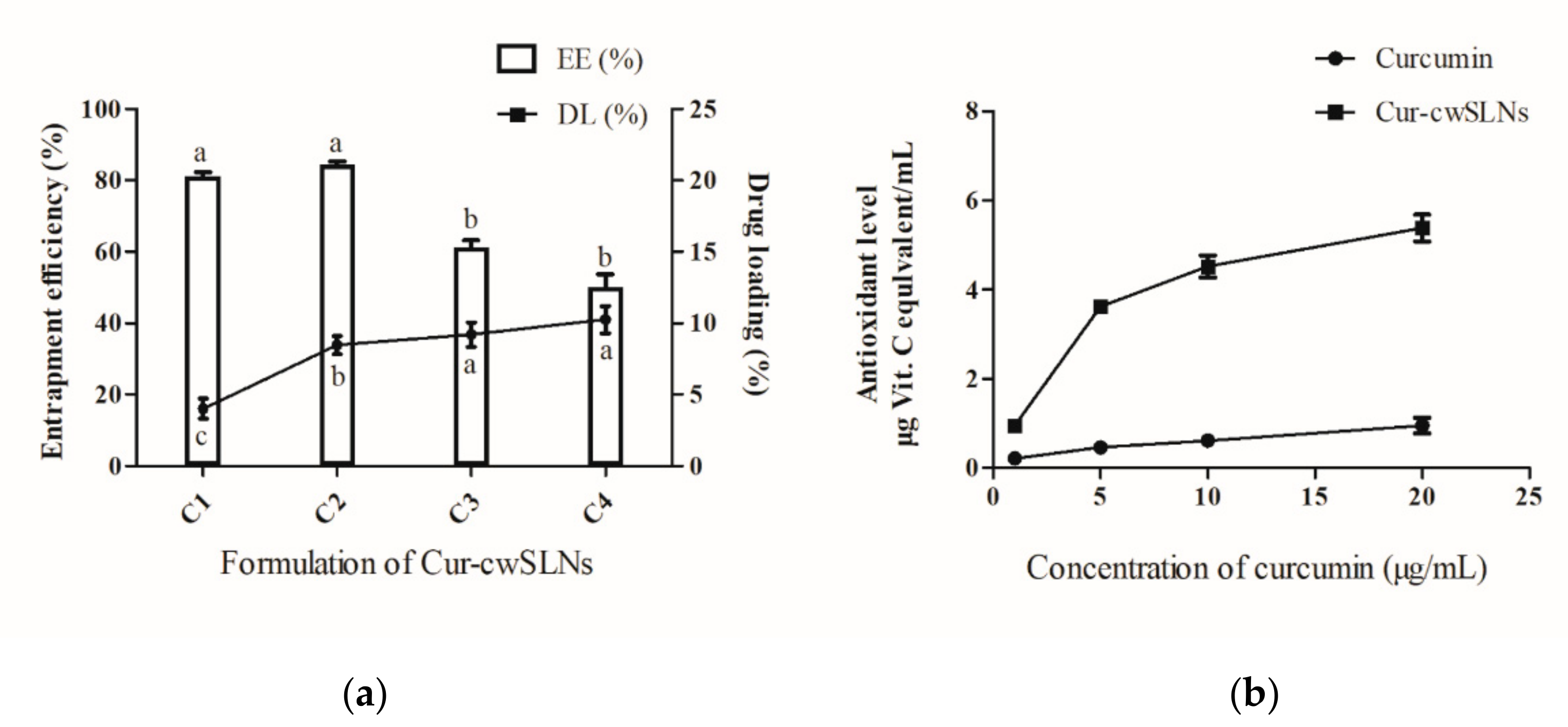
3.2. Characterization of Cur-cwSLNs
3.3. In Vitro Curcumin Release
3.4. In Vitro Cytotoxicity
3.5. In Vitro Anti-Biofilm Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siebra, A.L.A.; Oliveira, L.R.; Martins, A.O.B.P.B.; Siebra, D.C.; Albuquerque, R.S.; Lemos, I.C.S.; Delmondes, G.A.; Tintino, S.R.; Figueredo, F.G.; da Costa, J.G.M.; et al. Potentiation of antibiotic activity by Passiflora cincinnata Mast. front of strains Staphylococcus aureus and Escherichia coli. Saudi J. Biol. Sci. 2018, 25, 37–43. [Google Scholar] [CrossRef]
- Rittmann, B.E. Biofilms, active substrata, and me. Water Res. 2018, 132, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, K.X.; Ruan, L.; Chin, S.F.; Wickramasinghe, N.; Liu, H.B.; Ravikumar, V.; Ren, J.H.; Duan, H.W.; Yang, L.; et al. Block Copolymer Nanoparticles Remove Biofilms of Drug-Resistant Gram-Positive Bacteria by Nanoscale Bacterial Debridement. Nano Lett. 2018, 18, 4180–4187. [Google Scholar] [CrossRef] [PubMed]
- Cloete, T.E. Resistance mechanisms of bacteria to antimicrobial compounds. Int. Biodeterior. Biodegradation 2003, 51, 277–282. [Google Scholar] [CrossRef]
- Mi, G.J.; Shi, D.; Wang, M.; Webster, T.J. Reducing Bacterial Infections and Biofilm Formation Using Nanoparticles and Nanostructured Antibacterial Surfaces. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef]
- Ramos, M.A.D.S.; Da Silva, P.B.; Sposito, L.; De Toledo, L.G.; Bonifacio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Jenning, V.; Gohla, S. Comparison of wax and glyceride solid lipid nanoparticles (SLN). Int. J. Pharm. 2000, 196, 219–222. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, J.Y.; Gong, Z.J.; Xu, D.L.; Chen, X.M.; Liu, W.W.; Lin, X.D.; Li, Y.F. Transcriptome analysis of the Chinese white wax scale Ericerus pela with focus on genes involved in wax biosynthesis. PLoS ONE 2012, 7, e35719. [Google Scholar] [CrossRef]
- Sun, T.; Wang, X.Q.; Zhao, Z.L.; Yu, S.H.; Yang, P.; Chen, X.M. A Lethal Fungus Infects the Chinese White Wax Scale Insect and Causes Dramatic Changes in the Host Microbiota. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Murray, P.E.; Farber, R.M.; Namerow, K.N.; Kuttler, S.; Garcia-Godoy, F. Evaluation of Morinda citrifolia as an endodontic irrigant. J. Endod. 2008, 34, 66–70. [Google Scholar] [CrossRef]
- Neelakantan, P.; Cheng, C.Q.; Ravichandran, V.; Mao, T.; Sriraman, P.; Sridharan, S.; Subbarao, C.; Sharma, S.; Kishen, A. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal dentin. Photodiagnosis Photodyn. Ther. 2015, 12, 108–114. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed. Res. Int. 2014. [Google Scholar] [CrossRef]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Jourghanian, P.; Ghaffari, S.; Ardjmand, M.; Haghighat, S.; Mohammadnejad, M. Sustained release Curcumin loaded Solid Lipid Nanoparticles. Adv. Pharm. Bull. 2016, 6, 17–21. [Google Scholar] [CrossRef]
- Castellani, S.; Trapani, A.; Spagnoletta, A.; di Toma, L.; Magrone, T.; Di Gioia, S.; Mandracchia, D.; Trapani, G.; Jirillo, E.; Conese, M. Nanoparticle delivery of grape seed-derived proanthocyanidins to airway epithelial cells dampens oxidative stress and inflammation. J. Transl. Med. 2018, 16, 140. [Google Scholar] [CrossRef]
- Tan, Y.L.; Han, F.; Ma, S.; Yu, W.G. Carboxymethyl chitosan prevents formation of broad-spectrum biofilm. Carbohydr. Polym. 2011, 84, 1365–1370. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Zarei, H.; Golmohammadzadeh, S. Antibacterial efficacy of rifampin loaded solid lipid nanoparticles against Staphylococcus epidermidis biofilm. Microb. Pathog. 2016, 93, 137–144. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, J.S.; Park, J.K.; Lee, B.J.; Kim, M.S.; Hwang, S.J.; Lee, J.Y.; Cho, C.W. Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release. Molecules 2017, 22, 2215. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Kanaujia, P.; Kim, S.; Tan, R.B.H. Formulation design, preparation and physicochemical characterizations of solid lipid nanoparticles containing a hydrophobic drug: Effects of process variables. Colloids Surf. B Biointerfaces 2011, 88, 483–489. [Google Scholar] [CrossRef]
- Pandita, D.; Ahuja, A.; Velpandian, T.; Lather, V.; Dutta, T.; Khar, R.K. Characterization and in vitro assessment of paclitaxel loaded lipid nanoparticles formulated using modified solvent injection technique. Pharmazie 2009, 64, 301–310. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Hansen solubility parameters (HSP) for prescreening formulation of solid lipid nanoparticles (SLN): In vitro testing of curcumin-loaded SLN in MCF-7 and BT-474 cell lines. Pharm. Dev. Technol. 2018, 23, 96–105. [Google Scholar] [CrossRef]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Vivek, K.; Reddy, H.; Murthy, R.S.R. Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS PharmSciTech 2007, 8. [Google Scholar] [CrossRef]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Righeschi, C.; Bergonzi, M.C.; Isacchi, B.; Bazzicalupi, C.; Gratteri, P.; Bilia, A.R. Enhanced curcumin permeability by SLN formulation: The PAMPA approach. Lwt-Food Sci. Technol. 2016, 66, 475–483. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, S.L.; Zhu, L.Y.; Xie, S.Y.; Dong, Z.; Wang, Y.; Zhou, W.Z. Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Vet. J. 2012, 191, 115–120. [Google Scholar] [CrossRef]
- Malvajerd, S.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Del. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Mhule, D.; Kalhapure, R.S.; Jadhav, M.; Omolo, C.A.; Rambharose, S.; Mocktar, C.; Singh, S.; Waddad, A.Y.; Ndesendo, V.M.K.; Govender, T. Synthesis of an oleic acid based pH-responsive lipid and its application in nanodelivery of vancomycin. Int. J. Pharm. 2018, 550, 149–159. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ding, C.; Wang, Y.; Tan, H.; Li, J. pH-Responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater. Sci. 2019, 10. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed. Res. Int. 2018, 2018, 6498932. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Mocktar, C.; Sikwal, D.R.; Sonawane, S.J.; Kathiravan, M.K.; Skelton, A.; Govender, T. Ion pairing with linoleic acid simultaneously enhances encapsulation efficiency and antibacterial activity of vancomycin in solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 303–311. [Google Scholar] [CrossRef]
- Singh, B.; Vuddanda, P.R.; Vijayakumar, M.R.; Kumar, V.; Saxena, P.S.; Singh, S. Cefuroxime axetil loaded solid lipid nanoparticles for enhanced activity against S. aureus biofilm. Colloids Surf. B Biointerfaces 2014, 121, 92–98. [Google Scholar] [CrossRef]
- Ghaffari, S.; Varshosaz, J.; Saadat, A.; Atyabi, F. Stability and antimicrobial effect of amikacin-loaded solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 35–43. [Google Scholar] [CrossRef]
- Gupta, A.; Das, R.; Tonga, G.Y.; Mizuhara, T.; Rotello, V.M. Charge-Switchable Nanozymes for Bioorthogonal Imaging of Biofilm-Associated Infections. ACS Nano 2018, 12, 89–94. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Han, G.; Frases, S.; Cordero, R.J.B.; Casadevall, A.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. The use of chitosan to damage Cryptococcus neoformans biofilms. Biomaterials 2010, 31, 669–679. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef]





| Variables | Size (nm) | PDI | ZP (-mv) | |
|---|---|---|---|---|
| HT (min) | 20 | / | / | / |
| 30 | 729.4 ± 23.7 b | 0.538 ± 0.023 a | 23.9 ± 4.3 a,b | |
| 40 | 583.9 ± 30.2 c,d | 0.467 ± 0.043 b | 27.4 ± 3.2 a | |
| ST (min) | 4 | 626.6 ± 19.3 c | 0.428 ± 0.030 b,c | 25.7 ± 1.9 a,b |
| 6 | 532.7 ± 22.5 d | 0.376 ± 0.037 c | 24.1 ± 5.8 a,b | |
| 8 | 458.3 ± 25.0 e | 0.337 ± 0.029 c | 27.8 ± 4.2 a | |
| SC (%, w/v) | 1 | 664.6 ± 66.3 b,c | 0.361 ± 0.022 c | 19.2 ± 2.6 a,b |
| 3 | 450.8 ± 34.6 e | 0.332 ± 0.031 c | 24.8 ± 4.8 a,b | |
| 6 | 498.3 ± 31.6 d,e | 0.406 ± 0.048 b,c | 25.9 ± 5.1 a | |
| LC (%, w/v) | 0.5 | 950.7 ± 98.2 a | 0.553 ± 0. 023 a | 13.2 ± 8.2 b |
| 1 | 401.9 ± 21.3 f | 0.245 ± 0.018 d | 13.2 ± 8.2 b | |
| 1.5 | 475.8 ± 55.9 d,e | 0.402 ± 0.021 b,c | 20.4 ± 4.2 a,b |
| Curcumin Concentration | Average Particle Size (nm) | PDI |
|---|---|---|
| 5% | 411.8 ± 17.9 b | 0.241 ± 0.082 a |
| 10% | 423.7 ± 23.2 b | 0.310 ± 0.076 a |
| 15% | 476.3 ± 28.5 a | 0.192 ± 0.063 a |
| 20% | 498.5 ± 25.0 a | 0.218 ± 0.064 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, L.; Chi, Z.; Liu, C. Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms. Nanomaterials 2019, 9, 763. https://doi.org/10.3390/nano9050763
Luan L, Chi Z, Liu C. Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms. Nanomaterials. 2019; 9(5):763. https://doi.org/10.3390/nano9050763
Chicago/Turabian StyleLuan, Lin, Zhe Chi, and Chenguang Liu. 2019. "Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms" Nanomaterials 9, no. 5: 763. https://doi.org/10.3390/nano9050763
APA StyleLuan, L., Chi, Z., & Liu, C. (2019). Chinese White Wax Solid Lipid Nanoparticles as a Novel Nanocarrier of Curcumin for Inhibiting the Formation of Staphylococcus aureus Biofilms. Nanomaterials, 9(5), 763. https://doi.org/10.3390/nano9050763