Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospun PVDF Membranes
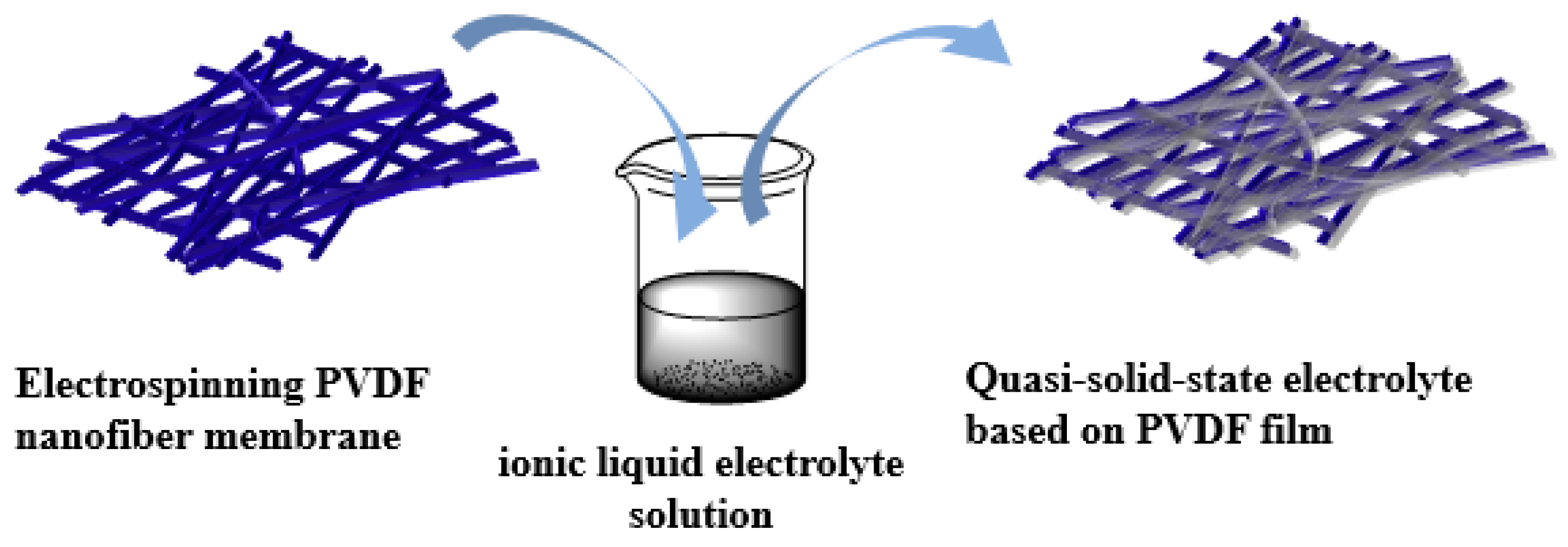
2.3. Preparation of the Quasi-Solid-State Electrolyte
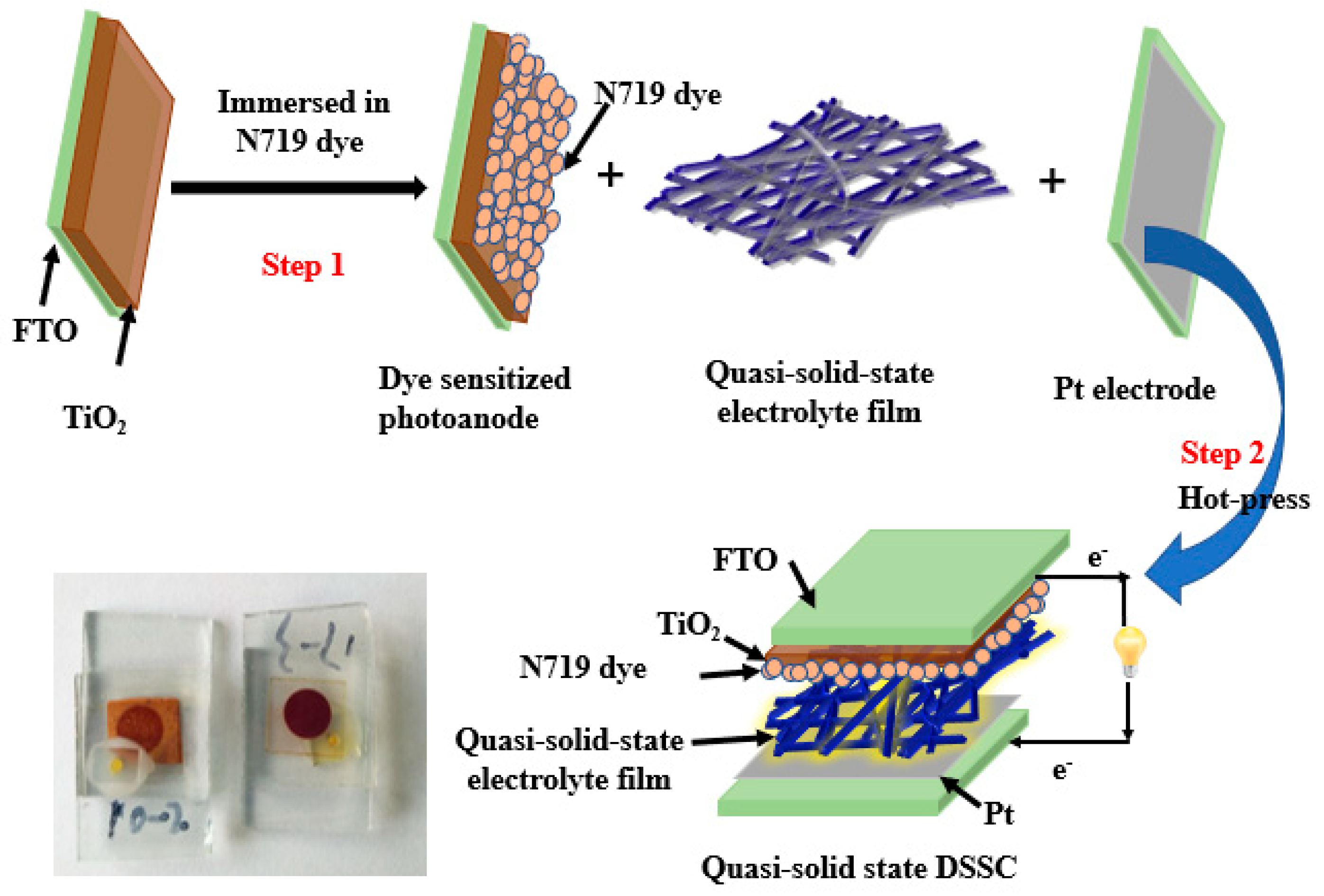
2.4. Fabrication of the Quasi-Solid-State DSSCs
2.5. Characterization
3. Results and Discussion
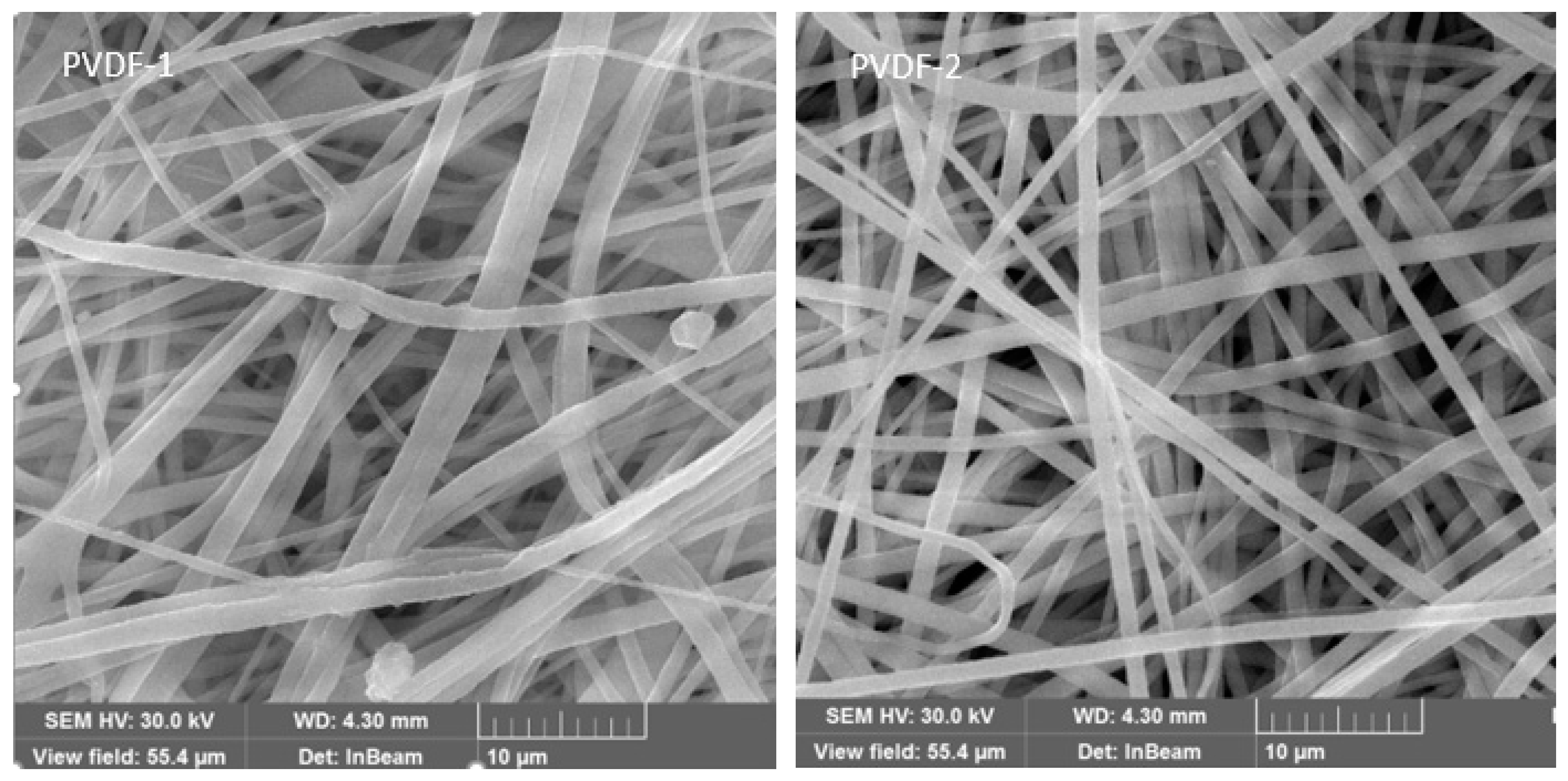
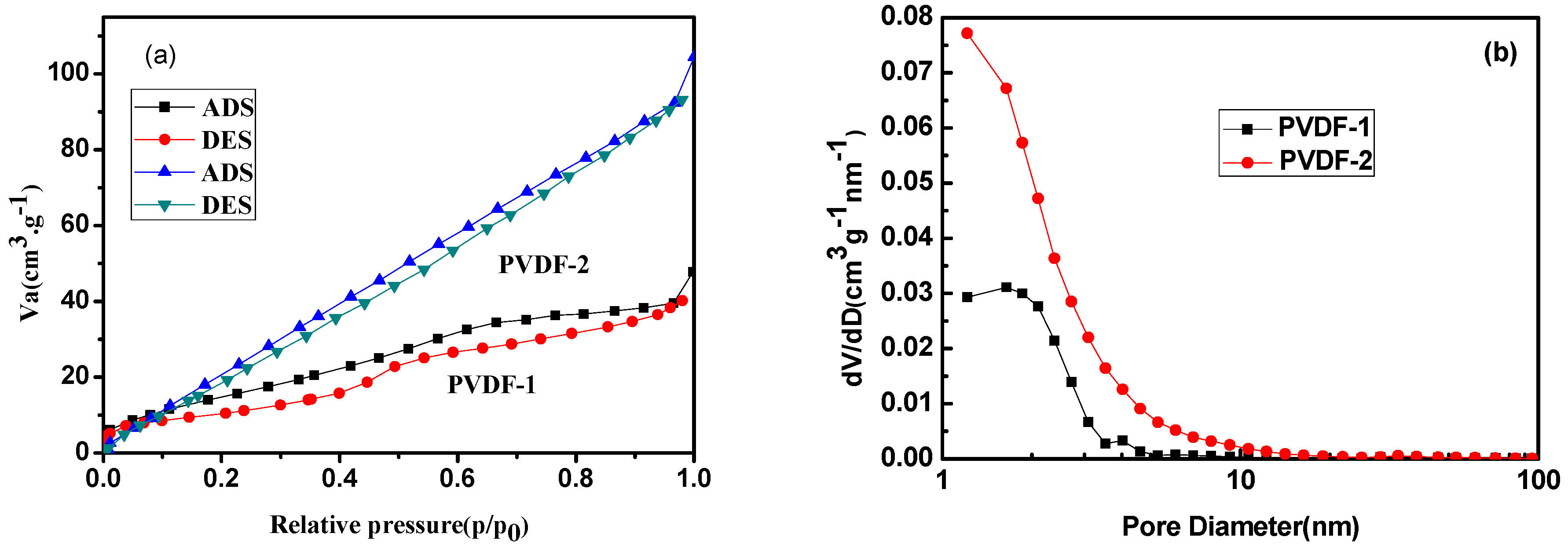
3.1. Electrospun PVDF Membrane
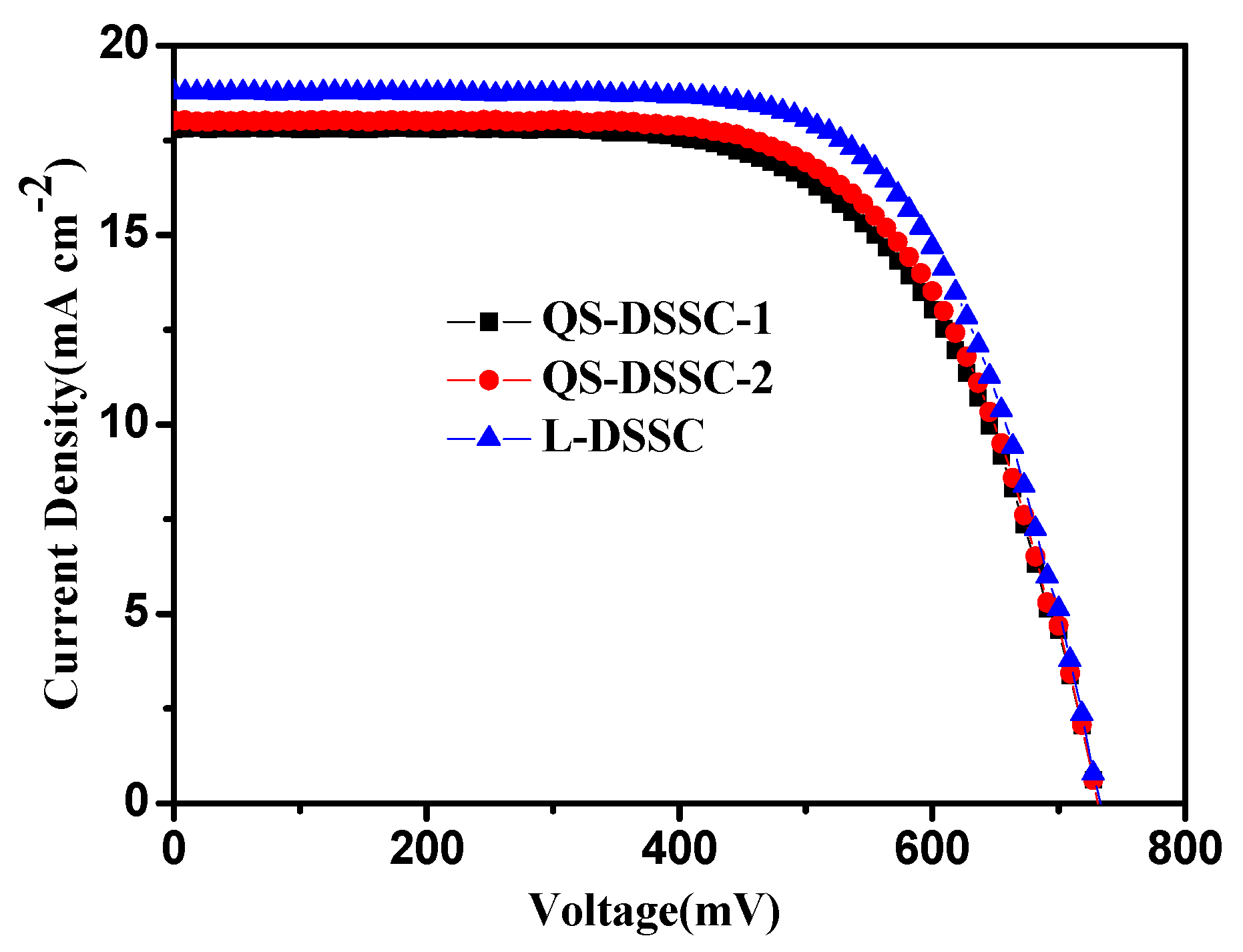
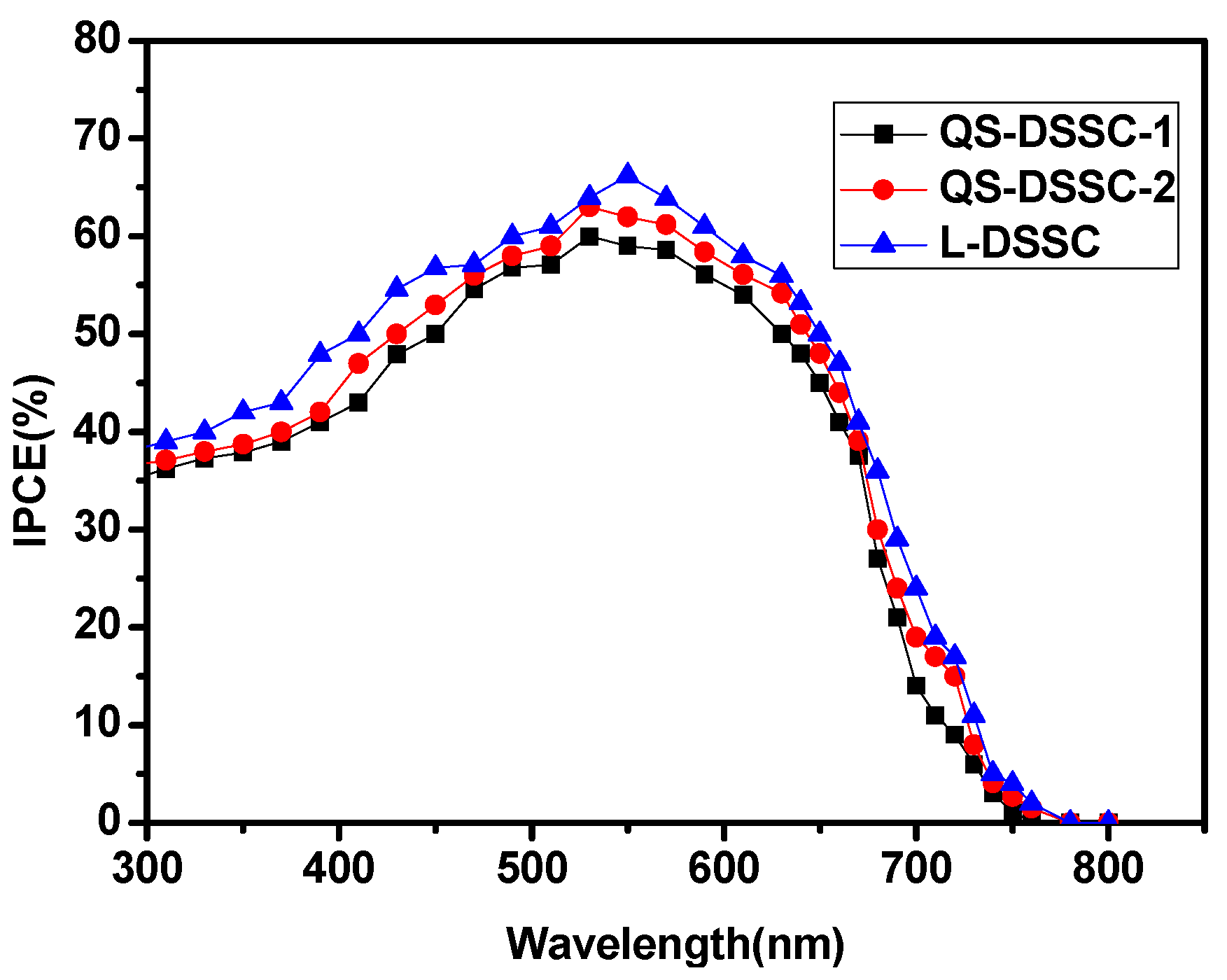
3.2. Photovoltaic Performance
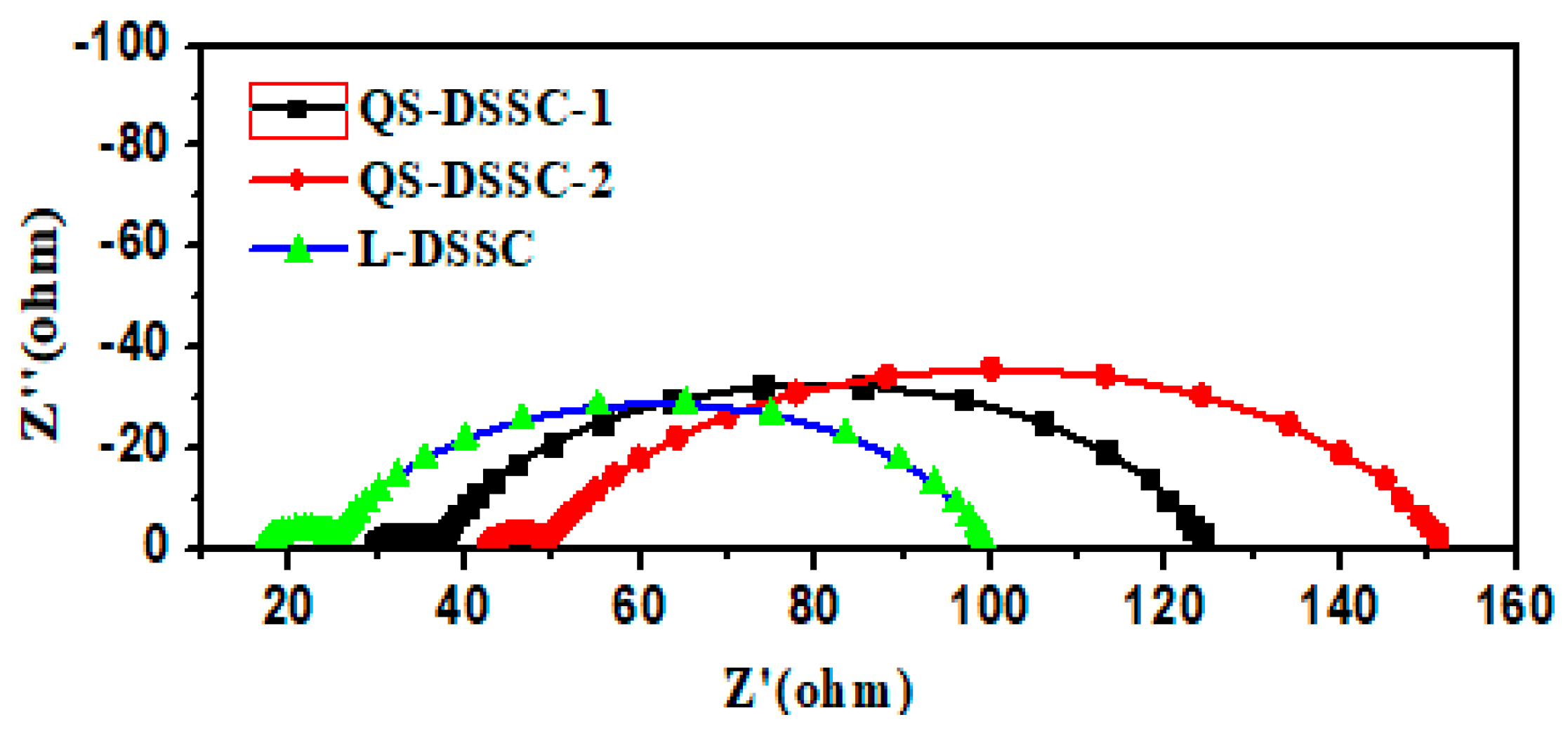
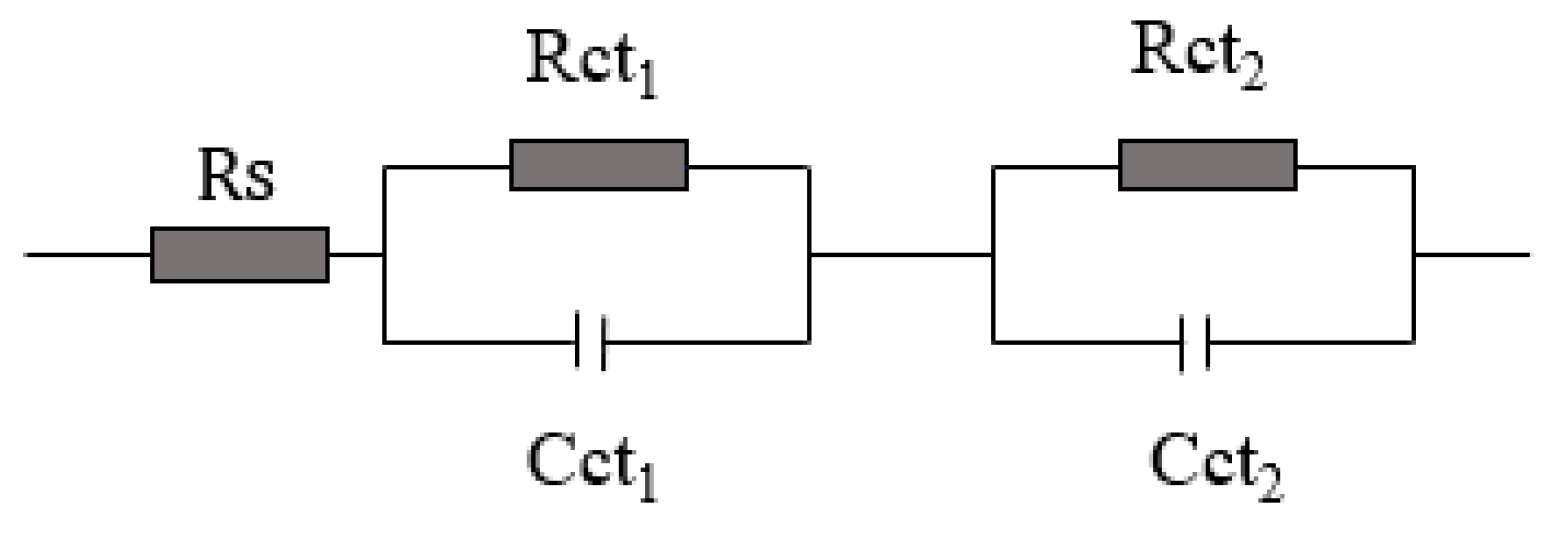
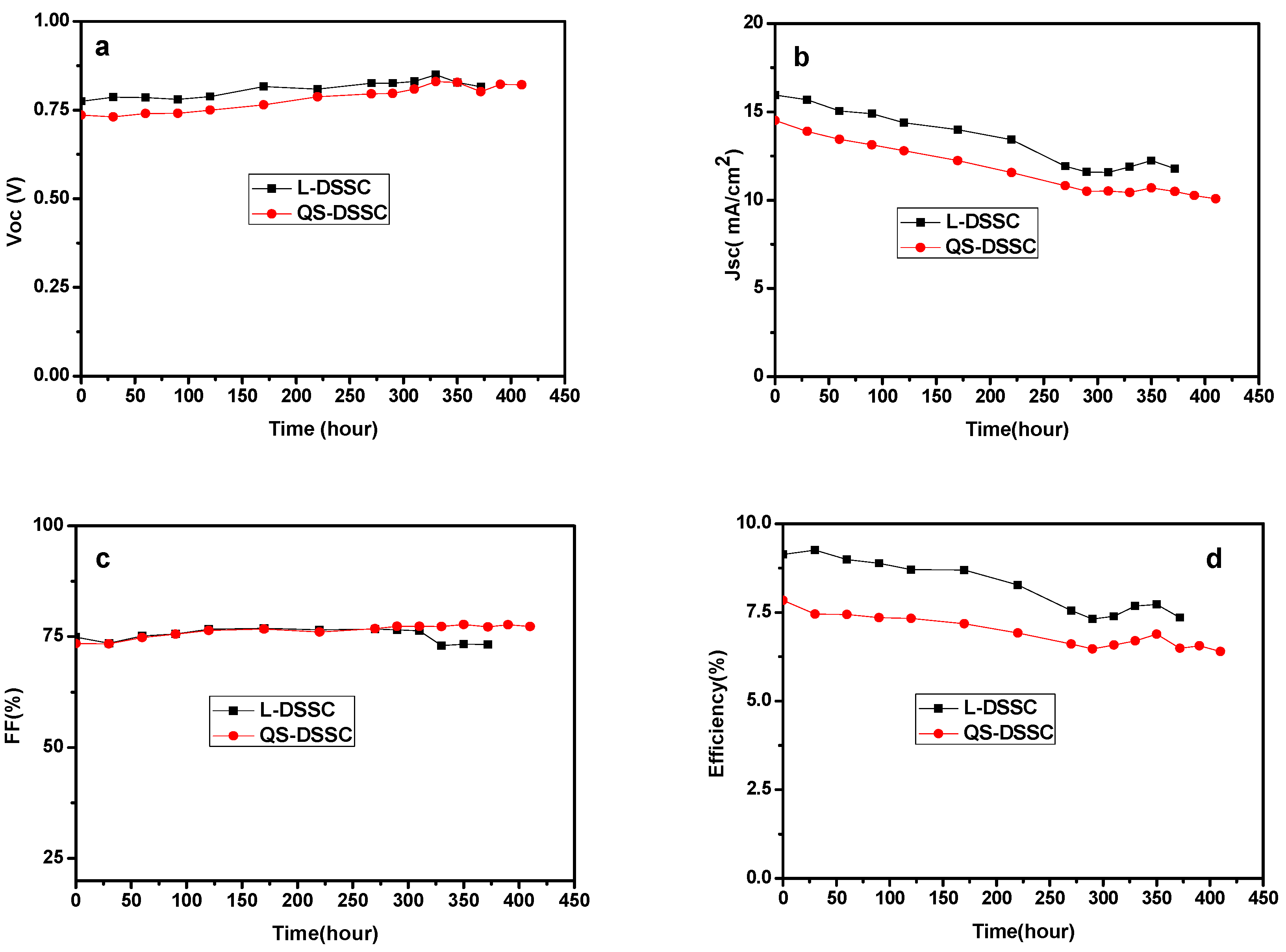
3.3. Stability of the Quasi-Solid-State DSSCs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su’ait, M.S.; Rahman, M.Y.A.; Ahmad, A. Review on polymer electrolyte in dye-sensitized solar cells (DSSCs). Sol. Energy 2015, 115, 452–470. [Google Scholar] [CrossRef]
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Mohan, K.; Bora, A.; Nath, B.C.; Gogoi, P.; Saikia, B.J.; Dolui, S.K. A highly stable and efficient quasi solid state dye sensitized solar cell based on Polymethylmethacrylate (PMMA)/Polyaniline Nanotube (PANI-NT) gel electrolyte. Electrochim. Acta 2016, 222, 1072–1078. [Google Scholar] [CrossRef]
- Yuan, S.S.; Tang, Q.W.; He, B.L.; Yang, P.Z. Efficient quasi-solid-state dye-sensitized solar cells employing polyaniline and polypyrrole incorporated microporous conducting gel electrolytes. J. Power Sources 2014, 254, 98–105. [Google Scholar] [CrossRef]
- Lee, R.H.; Cheng, T.F.; Chang, J.W.; Ho, J.H. Enhanced photovoltaic performance of quasi-solid-state dye-sensitized solar cells via incorporating quaternized ammonium iodide-containing conjugated polymer into PEO gel electrolytes. Colloid Polym. Sci. 2011, 289, 817–829. [Google Scholar] [CrossRef]
- Li, Q.H.; Chen, X.X.; Tang, Q.W.; Cai, H.Y.; Qin, Y.C.; He, B.L.; Li, M.J.; Jin, S.Y.; Liu, Z.C. Enhanced photovoltaic performances of quasi-solid-state dye sensitized solar cells using a novel conducting gel electrolyte. J. Power Sources 2014, 248, 923–930. [Google Scholar] [CrossRef]
- Anantharaj, G.; Joseph, J.; Selvaraj, M.; Jeyakumar, D. Fabrication of stable dye sensitized solar cell with gel electrolytes using poly (ethylene oxide)-poly (ethylene glycol). Electrochim. Acta 2015, 176, 1403–1409. [Google Scholar] [CrossRef]
- Aram, E.; Ehsani, M.; Khonakdar, H.A. Improvement of ionic conductivity and performance of quasi-solid-state dye sensitized solar cell using PEO/PMMA gel electrolyte. Thermochim. Acta 2015, 615, 61–67. [Google Scholar] [CrossRef]
- Tennakone, K.; Kumara, G.R.R.A.; Kumarasinghe, A.R.; Wijayantha, K.G.U.; Sirimanne, P.M. A dye-sensitized nano-porous solid-state photovoltaic cell. Semicond. Sci. Technol. 1995, 10, 689–1693. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T. Efficient photo-hole injection from adsorbed cyanine dyes into electrodeposited copper (I) thiocyanate thin films. Chem. Mater. 1995, 7, 1349–1354. [Google Scholar] [CrossRef]
- Kumara, G.R.R.; Konno, A.; Senadeera, G.K.R.; Jayaweera, P.V.V.; De Silva, D.B.R.A.; Tennakone, K. Dye-sensitized solar cell with the hole collector p-CuSCN deposited from a solution in n-propyl sulphide. Sol. Energy Mater. Sol. Cells. 2001, 69, 195–199. [Google Scholar] [CrossRef]
- O’Regan, B.; Schwartz, D.T. Large enhancement in photocurrent efficiency caused by UV illumination of the dye-sensitized heterojunction TiO2/RuLL NCS/CuSCN: Initiation and potential mechanisms. Chem. Mater. 1998, 10, 1501–1509. [Google Scholar] [CrossRef]
- Kavan, L.; Saygili, Y.; Freitag, M.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. Electrochemical properties of Cu(II/I)-based redox mediators for dye-sensitized solar cells. Electrochim. Acta 2017, 227, 194–202. [Google Scholar] [CrossRef]
- Schon, J.H.; Kloc, C.; Bucher, E.; Batlogg, B. Efficient organic photovoltaic diodes based on doped pentacene. Nature 2000, 403, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.E.; Brabec, C.J.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hummelen, J.C. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Liang, J. Polymer electrolyte based on polyethylene glycol for quasi-solid state dye sensitized solar cells. Renew. Energy 2012, 39, 419–423. [Google Scholar] [CrossRef]
- Lan, Z.; Wu, J.H.; Lin, J.M.; Huang, M.L. Quasi-solid-state dye-sensitized solar cells containing P (MMA-co-AN)-based polymeric gel electrolyte. Polym. Adv. Technol. 2011, 22, 1812–1815. [Google Scholar] [CrossRef]
- Arof, A.K.; Naeem, M.; Hameed, F.; Jayasundara, W.J.M.J.S.R.; Careem, M.A.; Teo, L.P.; Buraidah, M.H. Quasi solid state dye-sensitized solar cells based on polyvinyl alcohol (PVA) electrolytes containing I-/I3- redox couple. Opt. Quantum Electron. 2014, 46, 143–154. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Zhang, J.; Zhang, X.N.; Feng, J.W.; Hong, Y.; Zhu, Y.J. High performance quasi-solid-state dye-sensitized solar cells based on acetamide-modified polymer electrolytes. Org. Electron. 2012, 13, 2561–2567. [Google Scholar] [CrossRef]
- Zhao, J.X.; Jo, S.G.; Kim, D.W. Photovoltaic performance of dye-sensitized solar cells assembled with electrospun polyacrylonitrile/silica-based fibrous composite membranes. Electrochim. Acta 2014, 142, 261–267. [Google Scholar] [CrossRef]
- Park, S.H.; Won, D.H.; Choi, H.J.; Hwang, W.P.; Jang, S.I.; Kim, J.H.; Jeong, S.H.; Kim, J.U.; Lee, J.K.; Kim, M.R. Dye-sensitized solar cells based on electrospun polymer blends as electrolytes. Sol. Energy Mater. Sol. Cells 2011, 95, 296–300. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Divarathne, H.K.D.W.M.N.R.; Thotawatthage, C.A.; Dissanayake, C.B.; Senadeera, G.K.R.; Bandara, B.M.R. Dye-sensitized solar cells based on electrospun polyacrylonitrile (PAN) nanofibre membrane gel electrolyte. Electrochim. Acta 2014, 130, 76–81. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Subramania, A.; Fei, Z.F.; Dyson, P.J. Effect of 1-butyl-3-methylimidazolium iodide containing electrospun poly (vinylidene fluoride-co-hexafluoropropylene) membrane electrolyte on the photovoltaic performance of dye-sensitized solar cells. J. Appl. Polym. Sci. 2015, 132, 42032. [Google Scholar]
- Kim, J.U.; Park, S.H.; Choi, H.J.; Lee, W.K.; Lee, J.K.; Kim, M.R. Effect of electrolyte in electrospun poly(vinylidene fluoride-co-hexafluoropropylene) nanofibers on dye-sensitized solar cells. Sol. Energy Matrt. Sol. Cells 2009, 93, 803–807. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, H.J.; Lee, S.B.; Lee, S.M.; Cho, S.E.; Kim, K.H.; Kim, Y.K.; Kim, M.R.; Lee, J.K. Fabrications and photovoltaic properties of dye-sensitized solar cells with electrospun poly (vinyl alcohol) nanofibers containing Ag nanoparticles. Macromol. Res. 2011, 19, 142–146. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, H.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super-hydrophobic surface of aligned polyacrylonitrile nanofibers. Angew. Chem. Int. Ed. 2002, 41, 1221–1223. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-based synthesis of nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Ondarcuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. Europhys. Lett. 1998, 42, 215–220. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R. Poly (alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Gong, C.L.; Liu, H.; Zhang, B.Q.; Wang, G.J.; Cheng, F.; Zheng, G.W.; Wen, S.; Xue, Z.G.; Xie, X.L. High level of solid superacid coated poly (vinylidene fluoride) electrospun nanofiber composite polymer electrolyte membranes. J. Membr. Sci. 2017, 535, 113–121. [Google Scholar] [CrossRef]
- Lolla, D.; Lolla, M.; Abutale, A.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, polarization of electrospun polyvinylidene fluoride electret fibers and effect on capturing nanoscale solid aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef] [PubMed]


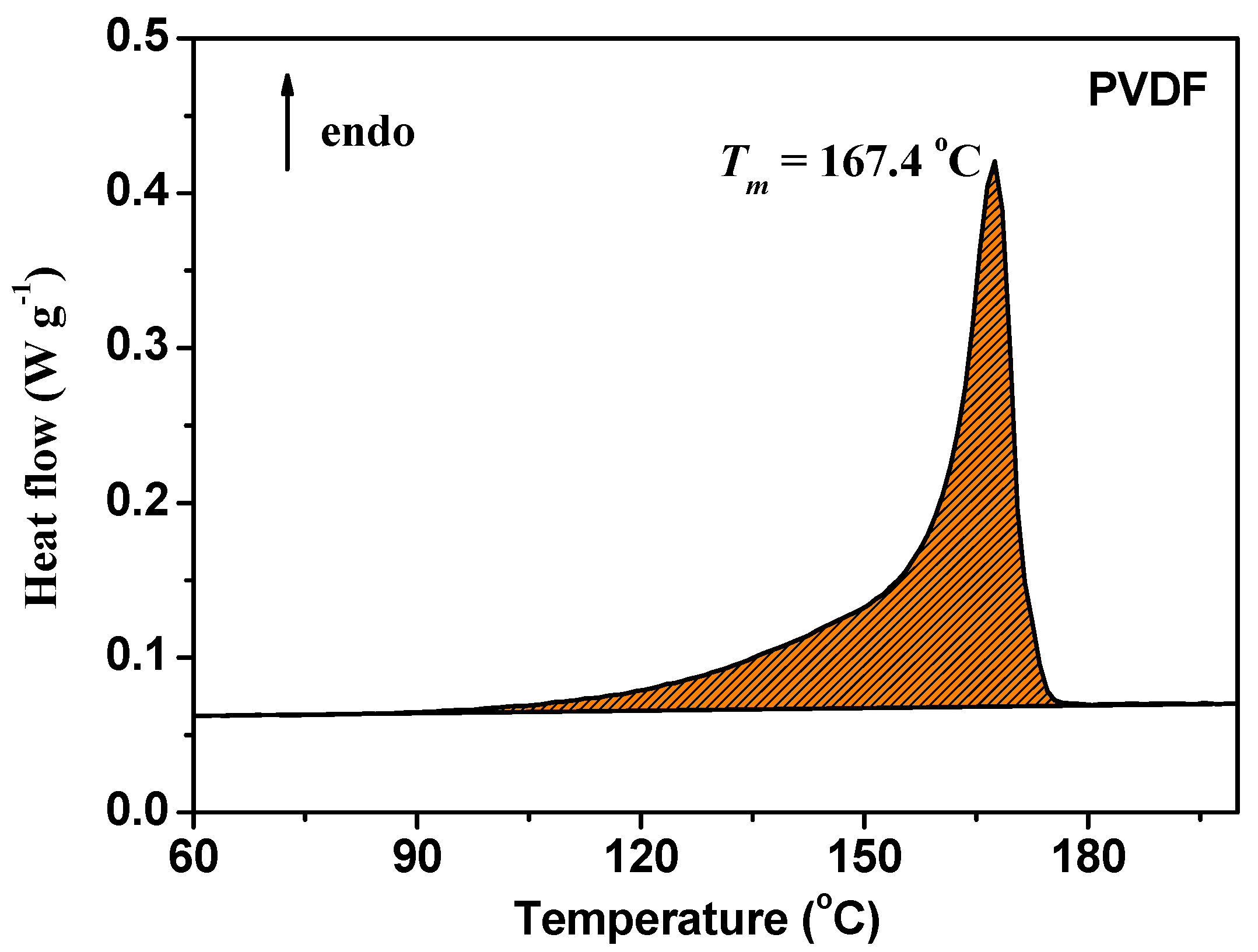


| Samples | Uptake Rate1 (%) | Uptake Rate2 (%) | Uptake Rate3 (%) | Average Uptake Rate (%) |
|---|---|---|---|---|
| PVDF-1 | 57 | 59 | 56 | 57.3 |
| PVDF-2 | 69 | 65 | 68 | 67.3 |
| Samples | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Mean Pore Diameter (nm) |
|---|---|---|---|
| PVDF-1 | 60.68 | 0.07 | 4.66 |
| PVDF-2 | 148.10 | 0.16 | 4.20 |
| Samples | η (%) | VOC (V) | JSC (mA.cm−2) | FF (%) |
|---|---|---|---|---|
| QS-DSSC-1 | 8.36 | 0.73 | 17.79 | 64 |
| QS-DSSC-2 | 8.63 | 0.73 | 18.01 | 66 |
| L-DSSC | 9.30 | 0.73 | 18.76 | 68 |
| Samples | RS | Rct1 | Rct2 |
|---|---|---|---|
| QS-DSSC-1 | 29.49 | 7.232 | 87.84 |
| QS-DSSC-2 | 42.37 | 6.622 | 103.1 |
| L-DSSC | 16.95 | 8.225 | 74.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.; Ou, Y.; Liu, G.; Zhao, L.; Dong, B.; Wang, S.; Wen, S. Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 783. https://doi.org/10.3390/nano9050783
Cheng F, Ou Y, Liu G, Zhao L, Dong B, Wang S, Wen S. Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable Dye-Sensitized Solar Cells. Nanomaterials. 2019; 9(5):783. https://doi.org/10.3390/nano9050783
Chicago/Turabian StyleCheng, Fan, Ying Ou, Guoliang Liu, Li Zhao, Binghai Dong, Shimin Wang, and Sheng Wen. 2019. "Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable Dye-Sensitized Solar Cells" Nanomaterials 9, no. 5: 783. https://doi.org/10.3390/nano9050783
APA StyleCheng, F., Ou, Y., Liu, G., Zhao, L., Dong, B., Wang, S., & Wen, S. (2019). Novel Quasi-Solid-State Electrolytes based on Electrospun Poly(vinylidene fluoride) Fiber Membranes for Highly Efficient and Stable Dye-Sensitized Solar Cells. Nanomaterials, 9(5), 783. https://doi.org/10.3390/nano9050783




