NiS2@rGO Nanosheet Wrapped with PPy Aerogel: A Sandwich-Like Structured Composite for Excellent Microwave Absorption
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of NiS2@rGO Binary Composite
2.3. Synthesis of NiS2@rGO/PPy Sandwich-Like Structure Composite
2.4. Characterization and Electromagnetic (EM) Parameters Measurement
2.5. EM Absorption Measurement
3. Results and Discussion
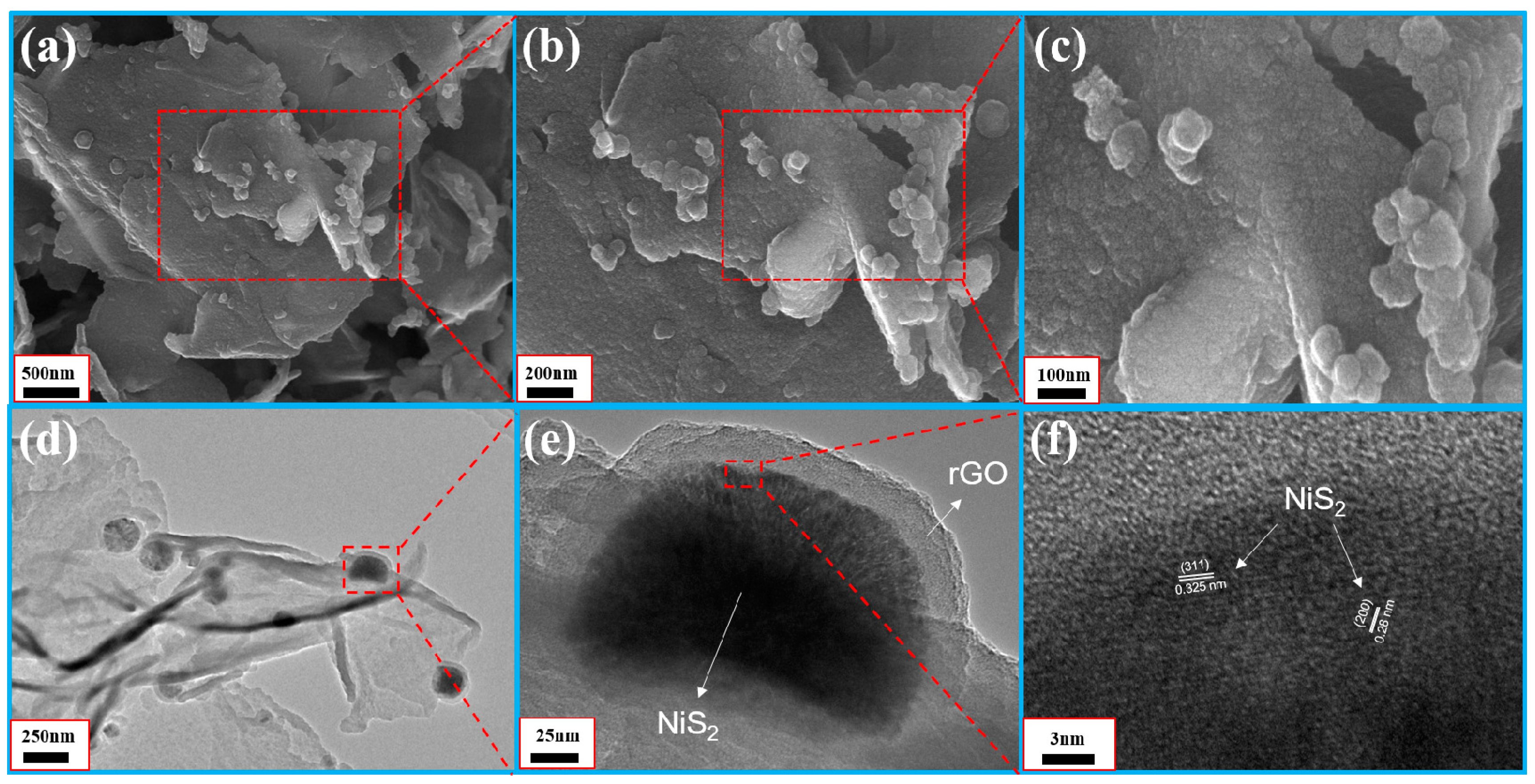
3.1. Characterization of Samples
3.2. Electromagnetic Parameters of the NiS2@rGO/PPy Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bahadur, A.; Saeed, A.; Iqbal, S.; Shoaib, M.; Ahmad, I.; Rahman, M.S.U.; Bashir, M.I.; Yaseen, M.; Hussain, W. Morphological and magnetic properties of BaFe12O19 nanoferrite: A promising microwave absorbing material. Ceram. Int. 2017, 43, 7346–7350. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Che, R.; Chen, H.; Liu, M.; Liu, Z. Hierarchical Fe3O4@TiO2 Yolk-Shell Microspheres with Enhanced Microwave-Absorption Properties. Chem. A Eur. J. 2013, 19, 6746–6752. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption. Adv. Mat. 2016, 28, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, F.; Xie, A.; Wang, Y. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar]
- Xia, T.; Zhang, C.; Oyler, N.A.; Chen, X. Hydrogenated TiO2 Nanocrystals: A Novel Microwave Absorbing Material. Adv. Mater. 2013, 25, 6905–6910. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, Z.; Zhang, D. Microwave absorbing property of a hybrid absorbent with carbonyl irons coating on the graphite. Appl. Surf. Sci. 2015, 356, 1032–1038. [Google Scholar] [CrossRef]
- Feng, J.; Hou, Y.; Wang, Y.; Li, L. Synthesis of Hierarchical ZnFe2O4@SiO2@RGO Core–Shell Microspheres for Enhanced Electromagnetic Wave Absorption. ACS Appl. Mater. Interfaces 2017, 9, 14103–14111. [Google Scholar] [CrossRef]
- He, J.; Li, G.; Sun, X.; Tang, J.; Wang, T.; Guo, Y.; Xue, H. Laminated magnetic graphene with enhanced electromagnetic wave absorption properties. J. Mater. Chem. C 2013, 1, 765–777. [Google Scholar]
- Tong, Y.; He, M.; Zhou, Y.; Nie, S.; Zhong, X.; Fan, L.; Huang, T.; Liao, Q.; Wang, Y. Three-Dimensional Hierarchical Architecture of the TiO2/Ti3C2Tx/RGO Ternary Composite Aerogel for Enhanced Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2018, 6, 8212–8222. [Google Scholar] [CrossRef]
- Wang, M.; Lin, M.; Li, J.; Huang, L.; Zhuang, Z.; Lin, C.; Zhou, L.; Mai, L. Metal–organic framework derived carbon-confined Ni2P nanocrystals supported on graphene for an efficient oxygen evolution reaction. Chem. Commun. 2017, 53, 8372–8375. [Google Scholar] [CrossRef]
- Wang, T.; Jia, X.; Li, Y.; Liu, C.; Geng, S.; Yang, F.; Zhang, L.; You, B.; Ren, X. Synthesis of graphene/α-Fe2O3 composites with excellent electromagnetic wave absorption properties. RSC Adv. 2015, 5, 60114–60120. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, A.; Wang, C.; Wang, H.; Shen, Y.; Tian, X. Novel rGO/α-Fe2O3 composite hydrogel: synthesis, characterization and high performance of electromagnetic wave absorption. J. Mater. Chem. A 2013, 1, 8547. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, X.; Xue, H.; Guo, H.; Fan, X.; Pan, X.; He, J. Graphene–carbonyl iron cross-linked composites with excellent electromagnetic wave absorption properties. J. Mater. Chem. C 2014, 2, 6582–6591. [Google Scholar] [CrossRef]
- Fang, S.; Huang, D.; Lv, R.; Bai, Y.; Huang, Z.-H.; Gu, J.; Kang, F. Three-dimensional reduced graphene oxide powder for efficient microwave absorption in the S-band (2–4 GHz). RSC Adv. 2017, 7, 25773–25779. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.; Xu, G.; Cheng, Y.; Zhang, Y.; Liu, W.; Ji, G.; Du, Y. A permittivity regulating strategy to achieve high-performance electromagnetic wave absorbers with compatibility of impedance matching and energy conservation. New J. Chem. 2017, 41, 1259–1266. [Google Scholar] [CrossRef]
- Sun, M.; Xie, A.; Zhang, K.; Jiang, W.; Wu, F.; He, M. In situ growth of MoS2 nanosheets on reduced graphene oxide (RGO) surfaces: interfacial enhancement of absorbing performance against electromagnetic pollution. Phys. Chem. Chem. Phys. 2016, 18, 24931–24936. [Google Scholar]
- Micheli, D.; Vricella, A.; Pastore, R.; Marchetti, M. Synthesis and electromagnetic characterization of frequency selective radar absorbing materials using carbon nanopowders. Carbon 2014, 77, 756–774. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Apollo, C.; Marchetti, M.; Gradoni, G.; Primiani, V.M.; Moglie, F. Broadband Electromagnetic Absorbers Using Carbon Nanostructure-Based Composites. IEEE Trans. Microw. Theory Tech. 2011, 59, 2633–2646. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Vricella, A.; Marchetti, M. Matter’s Electromagnetic Signature Reproduction by Graded-Dielectric Multilayer Assembly. IEEE Trans. Microw. Theory Tech. 2017, 65, 2801–2809. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, H.; Du, S. A facile hydrothermal synthesis of MnO2 nanorod–reduced graphene oxide nanocomposites possessing excellent microwave absorption properties. RSC Adv. 2015, 5, 88979–88988. [Google Scholar] [CrossRef]
- Zhao, T.; Ji, X.; Jin, W.; Wang, C.; Ma, W.; Gao, J.; Dang, A.; Li, T.; Shang, S.; Zhou, Z. Direct in situ synthesis of a 3D interlinked amorphous carbon nanotube/graphene/BaFe12O19 composite and its electromagnetic wave absorbing properties. RSC Adv. 2017, 7, 15903–15910. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, X.; He, N. Hierarchical nanostructured polypyrrole/graphene composites as supercapacitor electrode. RSC Adv. 2015, 5, 15096–15102. [Google Scholar]
- Que, R.; Fang, Y.; Gao, J.; Wang, X.; Shi, B. Hierarchical polypyrrole/Ni3S2@MoS2 core–shell nanostructures on a nickel foam for high-performance supercapacitors. RSC Adv. 2016, 6, 68460–68467. [Google Scholar]
- Xuan, Z.; Mao, C.; Li, G.; Guo, Z.; Zhong, Y.; Du, F. Polypyrrole-assisted synthesis of roselike MoS2/nitrogen-containing carbon/graphene hybrids and their robust lithium storage performances. RSC Adv. 2015, 5, 62624–62629. [Google Scholar]
- Liu, H.; Zhao, Q.; Wang, K.; Lu, Z.; Feng, F.; Guo, Y. Facile synthesis of polypyrrole nanofiber (PPyNF)/NiOx composites by a microwave method and application in supercapacitors. RSC Adv. 2019, 9, 6890–6897. [Google Scholar] [CrossRef]
- Yang, H.; Ye, T.; Lin, Y. Microwave absorbing properties based on polyaniline/magnetic nanocomposite powders. RSC Adv. 2015, 5, 103488–103493. [Google Scholar] [CrossRef]
- Chen, K.; Xiang, C.; Li, L.; Qian, H.; Xiao, Q.; Xu, F. A novel ternary composite: Fabrication, performance and application of expanded graphite/polyaniline/CoFe2O4 ferrite. J. Mater. Chem. 2012, 22, 6449. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Rehman, S.U.; Bi, H. Uniform core–shell PPy@carbon microsphere composites with a tunable shell thickness: the synthesis and their excellent microwave absorption performances in the X-band. RSC Adv. 2017, 7, 53104–53110. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhu, J.; Xu, J.; Liu, S.; Wang, Y.; Zhang, C.; Liu, T. A biomimetic Setaria viridis-inspired electrode with polyaniline nanowire arrays aligned on MoO3@polypyrrole core–shell nanobelts. J. Mater. Chem. A 2018, 6, 13428–13437. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714. [Google Scholar] [CrossRef]
- Li, Z.; Gong, F.; Zhou, G.; Wang, Z.-S. NiS2/Reduced Graphene Oxide Nanocomposites for Efficient Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 6561–6566. [Google Scholar] [CrossRef]
- Zhao, T.; Jin, W.; Wang, Y.; Ji, X.; Yan, H.; Xiong, C.; Lou, X.; Dang, A.; Li, H.; Li, T. In situ synthesis and electromagnetic wave absorbing properties of sandwich microstructured graphene/La-doped barium ferrite nanocomposite. RSC Adv. 2017, 7, 37276–37285. [Google Scholar] [CrossRef] [Green Version]
- ROSS, A.M.N.G.F. Measurement of the Intrinsic Properties of materials by Time-Domain Techniques. IEEE Trans. Instrum. Meas. 1970, 19, 377–382. [Google Scholar]
- WEIR, W.B. Automatic Measurement of Complex Dielectric Constant and Permeability at Microwave Frequencie. Proc. IEEE 1974, 62, 33–36. [Google Scholar] [CrossRef]
- Wang, X.C.; Shen, L.G.; Sun, Y.; Shu, Z.; Pei, Y.J.; Jin, K. Electromagnetic parameters study of microwaveabsorbing material FeSiAl for collinear load of LINAC. Conf. Proc. 2010, 100523, TUPEA075. [Google Scholar]
- Liu, P.; Huang, Y. Synthesis of reduced graphene oxide-conducting polymers-Co3O4 composites and their excellent microwave absorption properties. RSC Adv. 2013, 3, 19033. [Google Scholar] [CrossRef]
- Gu, X.; Yang, Y.; Hu, Y.; Hu, M.; Huang, J.; Wang, C. Facile fabrication of graphene–polypyrrole–Mn composites as high-performance electrodes for capacitive deionization. J. Mater. Chem. A 2015, 3, 5866–5874. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, X.; Chen, Y.; Zhang, P.; Sui, M.; Liu, H.; Sun, X. NiS2@MoS2 Nanospheres Anchored on Reduced Graphene Oxide: A Novel Ternary Heterostructure with Enhanced Electromagnetic Absorption Property. Nanomaterials 2019, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on reduced graphene oxide decorated with polypyrrole nanofibers and zinc oxide–copper oxide p–n junction heterostructures for the simultaneous voltammetric determination of ascorbic acid, dopamine, paracetamol, and tryptophan. New J. Chem. 2018, 42, 8512–8523. [Google Scholar]
- Rasouli, H.; Naji, L.; Hosseini, M. 3D structured polypyrrole/reduced graphene oxide (PPy/rGO)-based electrode ionic soft actuators with improved actuation performance. New J. Chem. 2018, 42, 12104–12118. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, S.-W.; Wang, G.-S.; Li, Z.; Guo, A.-P.; Zhu, J.-Q.; Liu, D.-P.; Yin, P.-G. Facile synthesis of NiS2@MoS2 core–shell nanospheres for effective enhancement in microwave absorption. RSC Adv. 2017, 7, 22454–22460. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, B.; Shao, G.; Xie, Y.; Zhang, R. Facile preparation and enhanced microwave absorption properties of core–shell composite spheres composited of Ni cores and TiO2 shells. Phys. Chem. Chem. Phys. 2015, 17, 8802–8810. [Google Scholar]
- Sun, X.; Yuan, X.; Li, X.; Li, L.; Song, Q.; Lv, X.; Gu, G.; Sui, M. Hollow cube-like CuS derived from Cu2O crystals for the highly efficient elimination of electromagnetic pollution. New J. Chem. 2018, 42, 6735–6741. [Google Scholar] [CrossRef]
- Sui, M.; Lu, X.; Xie, A.; Xu, W.; Rong, X.; Wu, G. The synthesis of three-dimensional (3D) polydopamine-functioned carbonyl iron powder@polypyrrole (CIP@PPy) aerogel composites for excellent microwave absorption. Synth. Met. 2015, 210, 156–164. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lv, Q.; Chen, Y.; Yu, H.; Liu, H.; Cui, G.; Sun, X.; Li, L. NiS2@rGO Nanosheet Wrapped with PPy Aerogel: A Sandwich-Like Structured Composite for Excellent Microwave Absorption. Nanomaterials 2019, 9, 833. https://doi.org/10.3390/nano9060833
Zhang Z, Lv Q, Chen Y, Yu H, Liu H, Cui G, Sun X, Li L. NiS2@rGO Nanosheet Wrapped with PPy Aerogel: A Sandwich-Like Structured Composite for Excellent Microwave Absorption. Nanomaterials. 2019; 9(6):833. https://doi.org/10.3390/nano9060833
Chicago/Turabian StyleZhang, Zhi, Qi Lv, Yiwang Chen, Haitao Yu, Hui Liu, Guangzhen Cui, Xiaodong Sun, and Ling Li. 2019. "NiS2@rGO Nanosheet Wrapped with PPy Aerogel: A Sandwich-Like Structured Composite for Excellent Microwave Absorption" Nanomaterials 9, no. 6: 833. https://doi.org/10.3390/nano9060833




