Regulation of Cu Species in CuO/SiO2 and Its Structural Evolution in Ethynylation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CuO/SiO2 Catalysts
2.1.1. Impregnation
2.1.2. Deposition and Precipitation
2.1.3. Ammonia Evaporation
2.2. Characterization of CuO/SiO2 Catalysts
2.3. Catalysis Tests of the CuO/SiO2 Catalysts
3. Results
3.1. Textural Properties of CuO/SiO2 Catalysts
3.2. Structural Analysis of CuO/SiO2 Catalysts
3.3. Morphological Analysis
3.4. FTIR Analysis
3.5. TG Analysis
3.6. Raman Spectra Analysis
3.7. XPS Characterization
3.8. X-Ray Auger Spectra Analysis of the Used Catalysts
3.9. CO-IR Spectra of the Used Catalysts
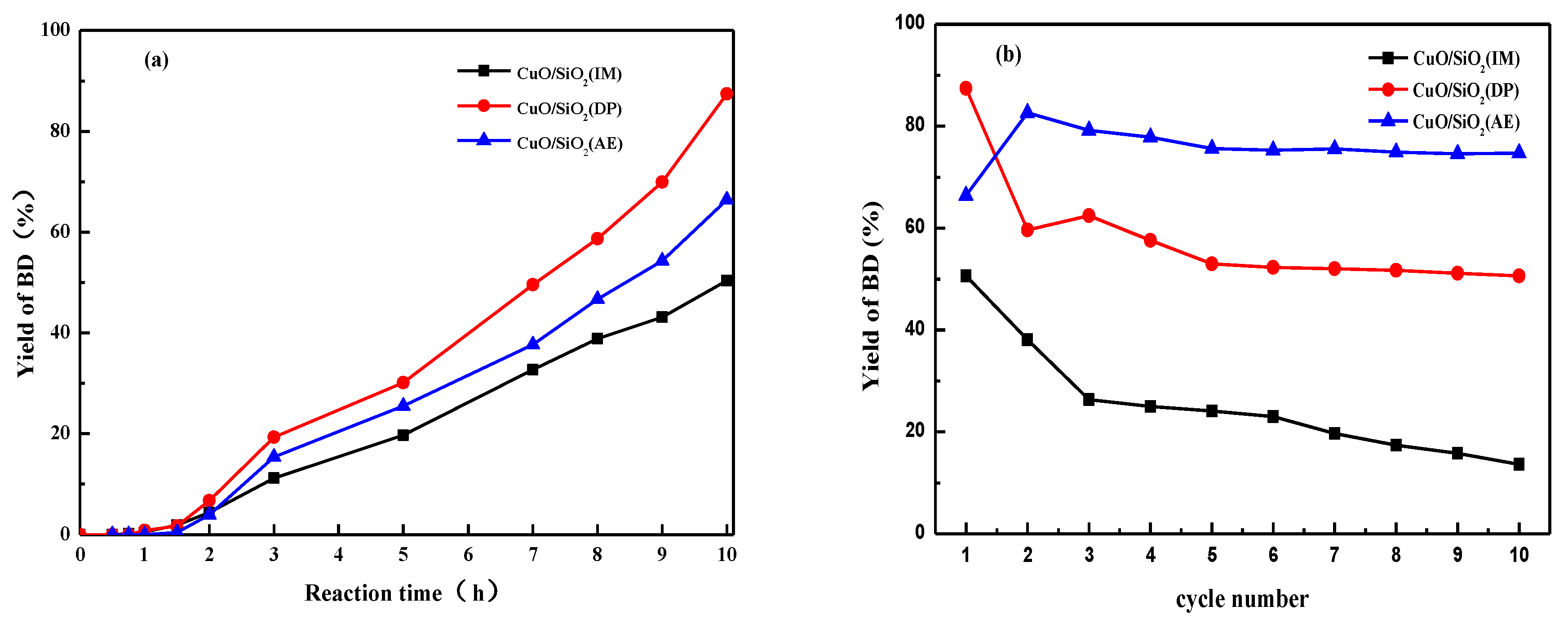
3.10. Catalytic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ge, Y.Y.; Jia, Z.Q.; Li, H.T. Amorphous aluminosilicates used as acid catalysts for tetrahydrofuran polymerization. React. Kinet. Mech. Catal. 2014, 112, 467–475. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.T.; Gao, C.G.; Zhao, Y.X. Heterogeneous CaO-ZrO2 acid-base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A Gen. 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Li, H.T.; Zhao, Y.X.; Gao, C.G.; Wang, Y.Z.; Sun, Z.J.; Liang, X.Y. Study on deactivation of Ni/Al2O3 catalyst for liquid phase hydrogenation of crude 1,4-butanediol aqueous solution. Chem. Eng. J. 2012, 181–182, 501–507. [Google Scholar] [CrossRef]
- Tanielyan, S.; Schmidt, S.; Marin, N.; Alvez, G.; Augustine, R. Selective hydrogenation of 2-Butyne-1,4-diol to 1,4-Butanediol over particulate Raney® Nickel catalysts. Top. Catal. 2010, 53, 1145–1149. [Google Scholar] [CrossRef]
- Chaudhari, R.V.; Rode, C.V.; Jaganathan, R. Process for the Conversion of 1,4-Butynediol of 1,4-Butenediol and 1,4-Butanediol. U.S. Patent 6469221B1, 16 July 2002. [Google Scholar]
- Zhou, S.L. Technology and Market Prospects of 1,4-Butanediol. Pet. Pet. Today 2014, 6, 35–40. [Google Scholar]
- Luo, M.; Li, H.T.; Ma, Z.Q.; Wang, J.J.; Zhao, Y.X. Researches on activation process of CuO-Bi2O3/SiO2-MgO catalyst in formaldehyde ethynylation reaction. Ind. Catal. (China) 2014, 22, 363–368. [Google Scholar]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New diol process: 1,3-propanediol and 1,4-butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- Zak, D.J. Butynediol Production. U.S. Patent 4085151, 18 April 1978. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol. U.S. Patent 4584418, 22 April 1986. [Google Scholar]
- Reppe, W.; Steinhofer, A.; Spaenig, H. Production of alkinols. U.S. Patent 2300969, 3 November 1942. [Google Scholar]
- Hort, E.V.; Piscataway, N.J. Ethynylation Catalyst and Method of Production Alkynols by Low Pressure Reactions. U.S. Patent 3920759, 18 November 1975. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol Using Bismuth Modified Spheroidal Malachite. U.S. Patent 4127734, 28 November 1978. [Google Scholar]
- Kirchner, R.J. Ethynylation of Formaldehyde. U.S. Patent 3560576, 2 February 1971. [Google Scholar]
- Bonrath, W.; Bernd, E.; Reinhard, K.; Schneider, M. Ethynylation Process. U.S. Patent 6949685, 27 September 2005. [Google Scholar]
- Trotuş, I.T.; Zimmermann, T.; Schuth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Z.J.; Wang, Y.Z.; Li, H.T.; Wang, S.A.; Luo, M.; Zhao, J.L.; Zhao, Y.X. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance. J. Mol. Catal. (China) 2012, 26, 233–238. [Google Scholar]
- Wang, J.J.; Li, H.T.; Ma, Z.Q.; Wang, Z.P.; Guo, J.Y.; Zhao, Y.X. Preparation of magnetic CuO-Bi2O3/Fe3O4-SiO2-MgO catalyst and its catalytic performance for formaldehyde ethynylation. J. Chem. Ind. Eng. 2015, 66, 2098–2104. [Google Scholar]
- Wang, Z.P.; Niu, Z.Z.; Ban, L.J.; Hao, Q.A.; Zhang, H.X.; Li, H.T.; Zhao, Y.X. Formaldehyde ethynylation reaction over Cu2O supported on TiO2 with different phases. Chem. J. Chin. Univ. 2019, 40, 334–341. [Google Scholar]
- Yang, G.F.; Li, H.T.; Zhang, H.X.; Wang, Z.P.; Liu, L.L.; Zhao, Y.X. Effect of NaOH concentration on structure and catalytic performance of Cu2O for formaldehyde ethynylation. J. Mol. Catal. (China) 2016, 30, 540–546. [Google Scholar]
- Li, H.T.; Niu, Z.Z.; Yang, G.F.; Zhang, H.X.; Wang, Z.P.; Zhao, Y.X. Support effect of Cu2O/TiO2 employed in formaldehyde ethynylation. J. Chem. Ind. Eng. 2018, 69, 2512–2518. [Google Scholar]
- Guerreiro, E.D.; Gorriz, O.F.; Larsen, G.; Arrua, L.A. Cu/SiO2 catalysts for methanol to methyl formate dehydrogenation: A comparative study using different preparation techniques. Appl. Catal. A 2000, 204, 33–48. [Google Scholar] [CrossRef]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester hydrogenolysis over promoted Cu/SiO2catalysts. Appl. Catal. A 1999, 184, 279–289. [Google Scholar] [CrossRef]
- Kasinathan, E.; Hwang, D.W.; Lee, U.H. Effect of Cu particle size on hydrogenation of dimethyl succinate over Cu-SiO2 nanocomposite. Catal. Commun. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol-gel methods. Appl. Catal. A 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Shan, S.F.; Liu, H.Y.; Shi, G.; Bao, X.J. Tuning of the active phase structure and hydrofining performance of alumina-supported tri-metallic WMoNi catalysts via phosphorus incorporation. Front. Chem. Sci. Eng. 2018, 12, 59–69. [Google Scholar] [CrossRef]
- Wang, Z.P.; Niu, Z.Z.; Hao, Q.A.; Ban, L.J.; Li, H.T.; Zhao, Y.X.; Jiang, Z. Enhancing the ethynylation performance of CuO-Bi2O3 nanocatalysts by tuning Cu-Bi interactions and phase structures. Catalysts 2019, 9, 35. [Google Scholar] [CrossRef]
- Lapisardi, G.; Chiker, F.; Launay, F.; Nogier, J.-P.; Bonardet, J.-L. A “one-pot” synthesis of adipic acid from cyclohexene under mild conditions with new bifunctional Ti-AlSBA mesostructured catalysts. Catal. Commun. 2004, 5, 277–281. [Google Scholar] [CrossRef]
- Yao, Q.L.; Lu, Z.H.; Wang, Y.Q.; Chen, X.S.; Feng, G. Synergetic Catalysis of Non-noble Bimetallic Cu-Co Nanoparticles Embedded in SiO2 Nanospheres in Hydrolytic Dehydrogenation of Ammonia Borane. J. Phys. Chem. C 2015, 119, 14167–14174. [Google Scholar] [CrossRef]
- Wang, S.; Guo, W.W.; Wang, H.X.; Zhu, L.J.; Yin, S.; Qiu, K.Z. Effect of the Cu/SBA-15 catalyst preparation method on methyl acetate hydrogenation for ethanol production. New J. Chem. 2014, 38, 2792. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Xu, Z.N.; Peng, S.Y.; Zhang, M.J.; Lu, G.; Chen, Q.S.; Chen, Y.M.; Guo, G.C. High-Performance and Long-Lived Cu/SiO2 Nanocatalyst for CO2 Hydrogenation. ACS Catal. 2015, 5, 4255–4259. [Google Scholar] [CrossRef]
- Van der Grift, C.J.G.; Wielers, A.F.H.; Mulder, A.; Geus, J.W. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation. Thermochim. Acta 1990, 171, 95. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Diaz, G.; Perez-Hernandez, R.; Gómez-Cortés, A.; Benaissa, M.; Mariscal, R.; Fierro, J. CuO–SiO2 Sol–Gel Catalysts: Characterization and Catalytic Properties for NO Reduction. J. Catal. 1999, 187, 1–14. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.-F.; Louis, C. Conditions of Formation of Copper Phyllosilicates in Silica-Supported Copper Catalysts Prepared by Selective Adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Cordoba, G.; Arroyo, R.; Fierro, J.L.G.; Viniegra, M. Study of xerogel-glass transition of CuO/SiO2. J. Solid State Chem. 1996, 123, 93–99. [Google Scholar] [CrossRef]
- Huang, Z.W.; Cui, F.; Xue, J.J.; Zuo, J.L. Cu/SiO2 catalysts prepared by hom-and heterogeneous deposition-precipitation methods: Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Today 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Ischenko, E.V.; Matzui, L.Y.; Gayday, S.V.; Vovchenko, L.L.; Kartashova, T.V.; Lisnyak, V.V. Thermo-Exfoliated Graphite Containing CuO/Cu2(OH)3NO3:(Co2+/Fe3+) Composites: Preparation, Characterization and Catalytic Performance in CO Conversion. Materials 2010, 3, 572–584. [Google Scholar] [CrossRef]
- Yurieva, T.M.; Kustova, G.N.; Minyukova, T.P.; Poels, E.K.; Bliek, A.; Demeshkina, M.P.; Plyasova, L.M.; Krieger, T.A.; Zaikovskii, V.I. Non-hydrothermal synthesis of copper-, zinc-and copper-zinc hydrosilicates. Mater. Res. Innov. 2001, 5, 3–11. [Google Scholar] [CrossRef]
- Goldstein, H.F.; Kim, D.S.; Yu, P.Y.; Bourne, L.C. Raman study of CuO single crystals. Phys. Rev. B 1990, 41, 7192. [Google Scholar] [CrossRef]
- Irwin, J.C.; Chrzanowski, J.; Wei, T.; Lockwood, D.J.; Wold, A. Raman scattering from single crystals of cupric oxide. Physica C 1990, 166, 456–464. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Liu, Y.; Xu, C.; Zheng, C.; Wang, G. A simple wet-chemical synthesis and characterization of CuO nanorods. Appl. Phys. A 2003, 76, 417–420. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Li, W.S.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Preparation and Characterization of CuO Nanocrystals. J. Raman Spectrosc. 1999, 30, 413–415. [Google Scholar] [CrossRef]
- Raimondi, F.; Geissler, K.; Wambach, J.; Wokaun, A. Hydrogen production by methanol reforming: Post-reaction characterisation of a Cu/ZnO/Al2O3 catalyst by XPS and TPD. Appl. Surf. Sci. 2002, 189, 59–71. [Google Scholar] [CrossRef]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2 Catalysts with Balanced Cu0–Cu+ Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef]
- Ding, J.; Popa, T.; Tang, J. Highly selective and stable Cu/SiO2 catalysts prepared with a green method for hydrogenation of diethyl oxalate into ethylene glycol. Appl. Catal. B Environ. 2017, 209, 530–542. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, H.; Cui, F.; Kang, H.; Chen, J.; Zou, J.; Xia, C. Effects of the precipitation agents and rare earth additives on the structure and catalytic performance in glycerol hydrogenolysis of Cu/SiO2 catalysts prepared by precipitation-gel method. Catal. Today 2014, 234, 223–232. [Google Scholar] [CrossRef]
- Gervasini, A.; Manzoli, M.; Martra, G.; Ponti, A.; Ravasio, N.; Sordelli, L.; Zaccheria, F. Dependence of Copper Species on the Nature of the Support for Dispersed CuO Catalysts. J. Phys. Chem. B 2006, 110, 7851–7861. [Google Scholar] [CrossRef]
- Auroux, A.; Gervasini, A.; Guimon, C. Acidic Character of Metal-Loaded Amorphous and Crystalline Silica−Aluminas Determined by XPS and Adsorption Calorimetry. J. Phys. Chem. B 1999, 103, 7195–7205. [Google Scholar] [CrossRef]
- Chang, F.W.; Kuo, W.Y.; Lee, K.C. Dehydrogenation of ethanol over copper catalysts on rice husk ash prepared by incipient wetness impregnation. Appl. Catal. A 2003, 246, 253–264. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Q.; Wang, X.; Ma, K.; Bai, X.; Tan, S.; Tian, Y.; Ding, T.; Zheng, L.; Zhang, J.; et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals. Appl. Surf. Sci. 2017, 422, 932–943. [Google Scholar] [CrossRef]
- Hornes, A.; Bera, P.; Camara, A.L.; Gamarra, D.; Munuera, G.; Martinez-Arias, A. CO-TPR-DRIFTS-MS in situ study of CuO/Ce1−xTbxO2−y (x = 0, 0.2 and 0.5) catalysts: Support effects on redox properties and CO oxidation catalysis. J. Catal. 2009, 268, 367–375. [Google Scholar] [CrossRef]
- Manzoli, M.; Monte, R.D.; Boccuzzi, F.; Coluccia, S.; Kašpar, J. CO oxidation over CuOx-CeO2-ZrO2 catalysts: Transient behaviour and role of copper clusters in contact with ceria. Appl. Catal. B 2005, 61, 192–205. [Google Scholar] [CrossRef]
- Guo, X.L.; Zhou, R.X. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ray, P.; Gong, Q.H.; Zhao, Y.G.; Li, J.; Lueking, A.D. Influence of gas packing and orientation on FTIR activity for CO chemisorption to the Cu paddlewheel. Phys. Chem. Chem. Phys. 2015, 17, 26766. [Google Scholar] [CrossRef] [PubMed]
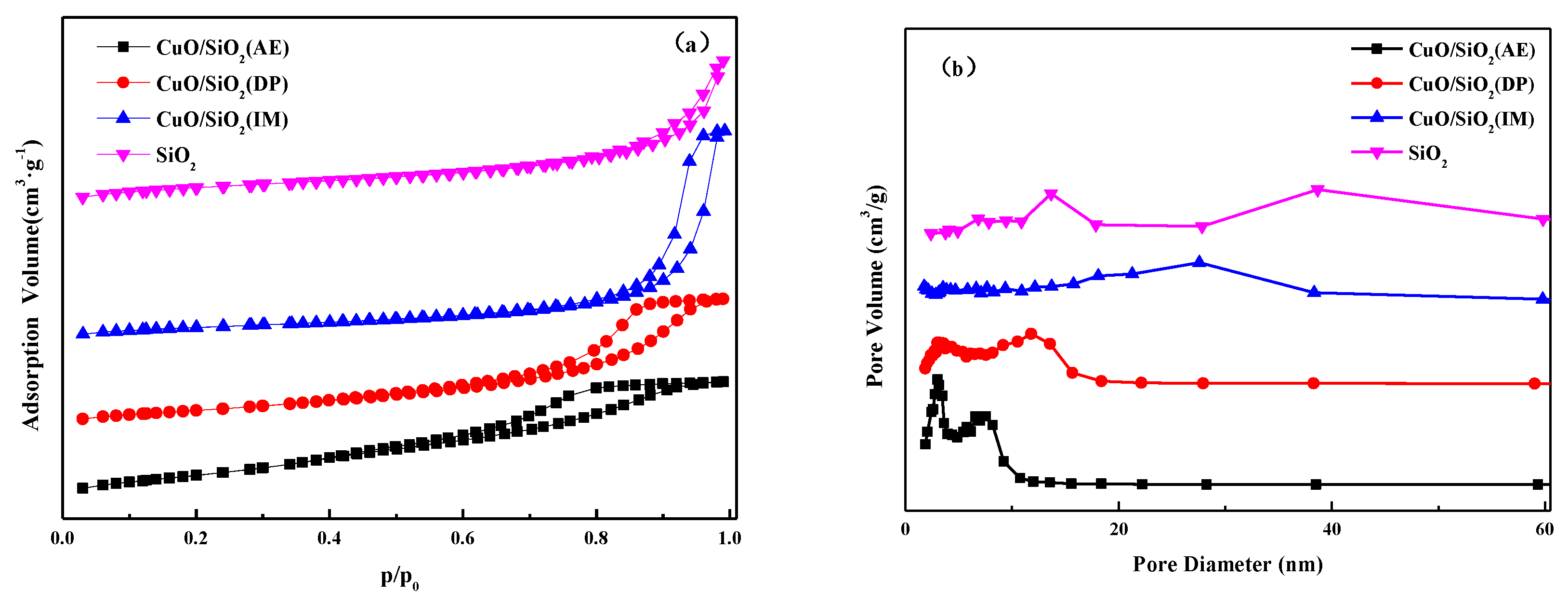


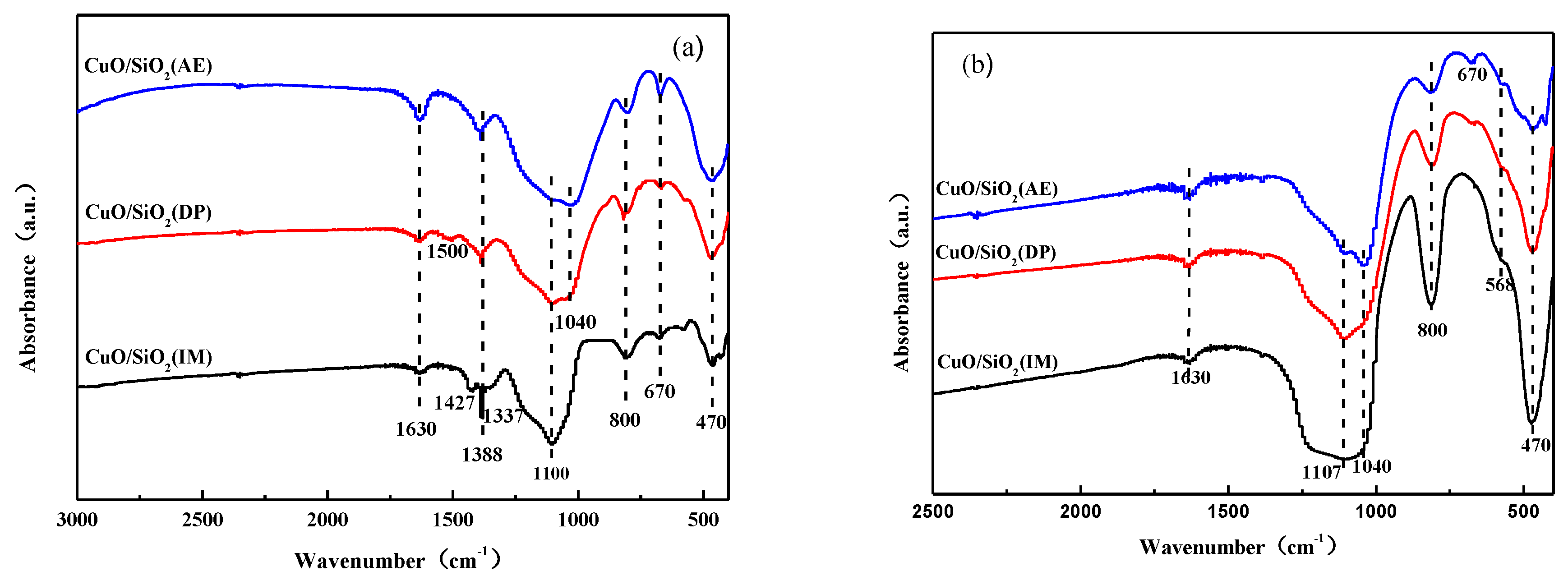


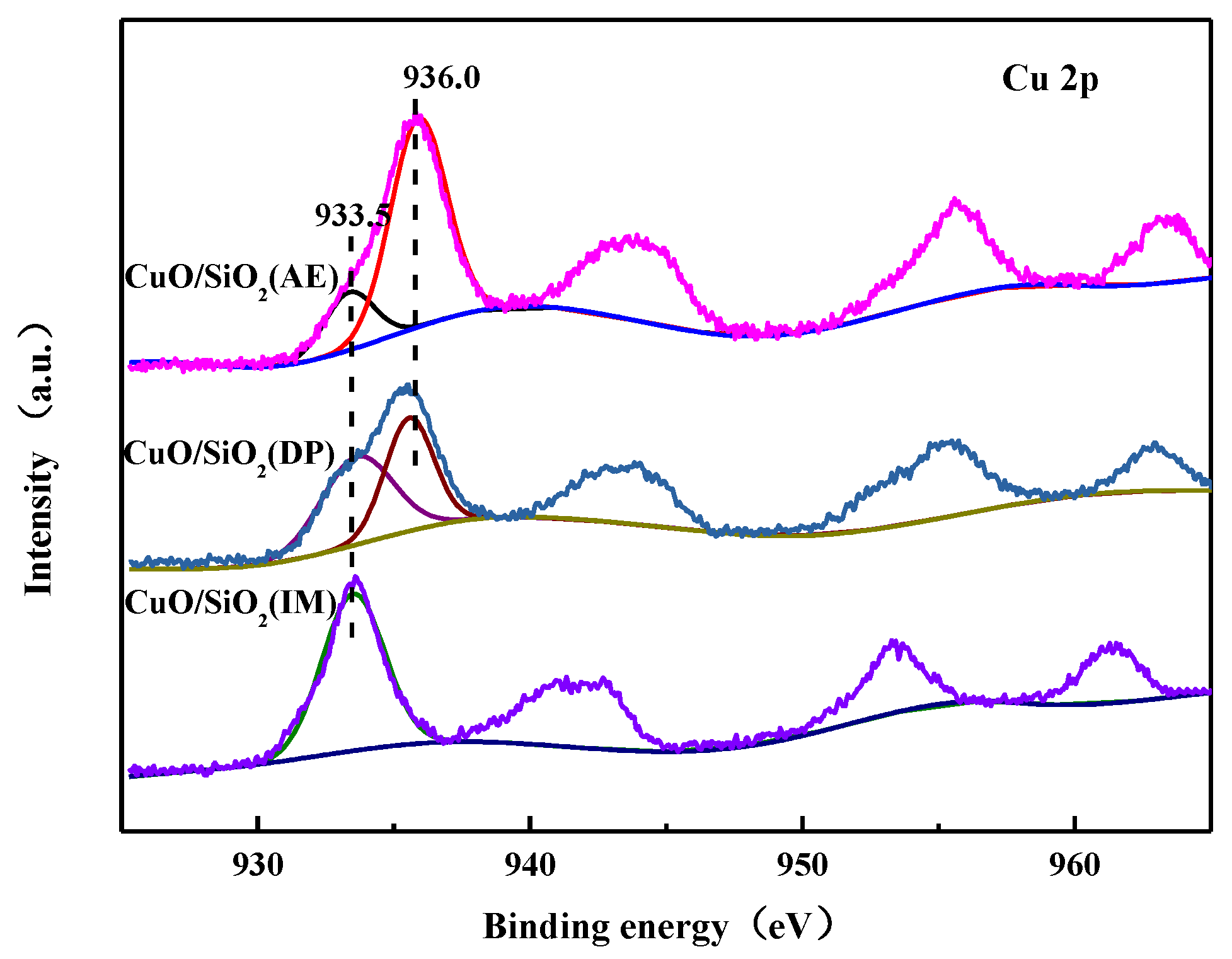

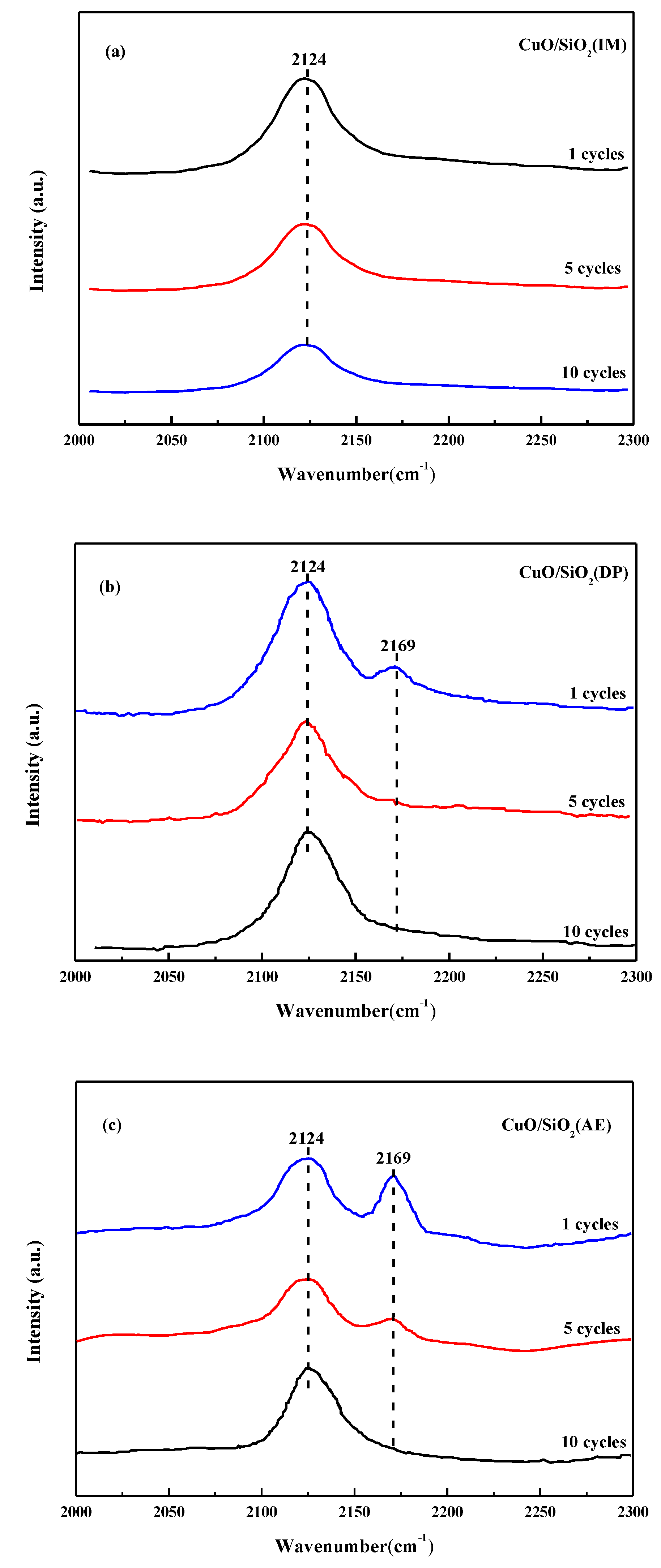

| Catalysts | SBET (m2·g−1) | Dpore (nm) | Vpore (cm3·g−1) | Grain Size a (nm) |
|---|---|---|---|---|
| CuO/SiO2(IM) | 231 | 17.4 | 1.07 | 16.2 |
| CuO/SiO2(DP) | 304 | 7.1 | 0.69 | 7.6 |
| CuO/SiO2(AE) | 457 | 4.6 | 0.67 | − |
| SiO2 | 328 | 9.3 | 0.77 | − |
| Catalyst | Cu in Mother Liquid (mg/L) | ||
|---|---|---|---|
| 1 Cycle | 5 Cycles | 10 Cycles | |
| CuO/SiO2(IM) | 85.3 | 87.6 | 89.3 |
| CuO/SiO2(DP) | 60.1 | 64.7 | 66.4 |
| CuO/SiO2(AE) | 35.8 | 38.2 | 36.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ban, L.; Wang, Z.; Meng, P.; Zhang, Y.; Wu, R.; Zhao, Y. Regulation of Cu Species in CuO/SiO2 and Its Structural Evolution in Ethynylation Reaction. Nanomaterials 2019, 9, 842. https://doi.org/10.3390/nano9060842
Li H, Ban L, Wang Z, Meng P, Zhang Y, Wu R, Zhao Y. Regulation of Cu Species in CuO/SiO2 and Its Structural Evolution in Ethynylation Reaction. Nanomaterials. 2019; 9(6):842. https://doi.org/10.3390/nano9060842
Chicago/Turabian StyleLi, Haitao, Lijun Ban, Zhipeng Wang, Pingfan Meng, Yin Zhang, Ruifang Wu, and Yongxiang Zhao. 2019. "Regulation of Cu Species in CuO/SiO2 and Its Structural Evolution in Ethynylation Reaction" Nanomaterials 9, no. 6: 842. https://doi.org/10.3390/nano9060842





