In-Depth Characterization of Secondary Phases in Cu2ZnSnS4 Film and Its Application to Solar Cells
Abstract
:1. Introduction
2. Experimental Approach
3. Results and Discussion
3.1. Characterization of the Crystallization of a CZTS Film
3.2. In-Depth Elemental Distribution in a CZTS Film
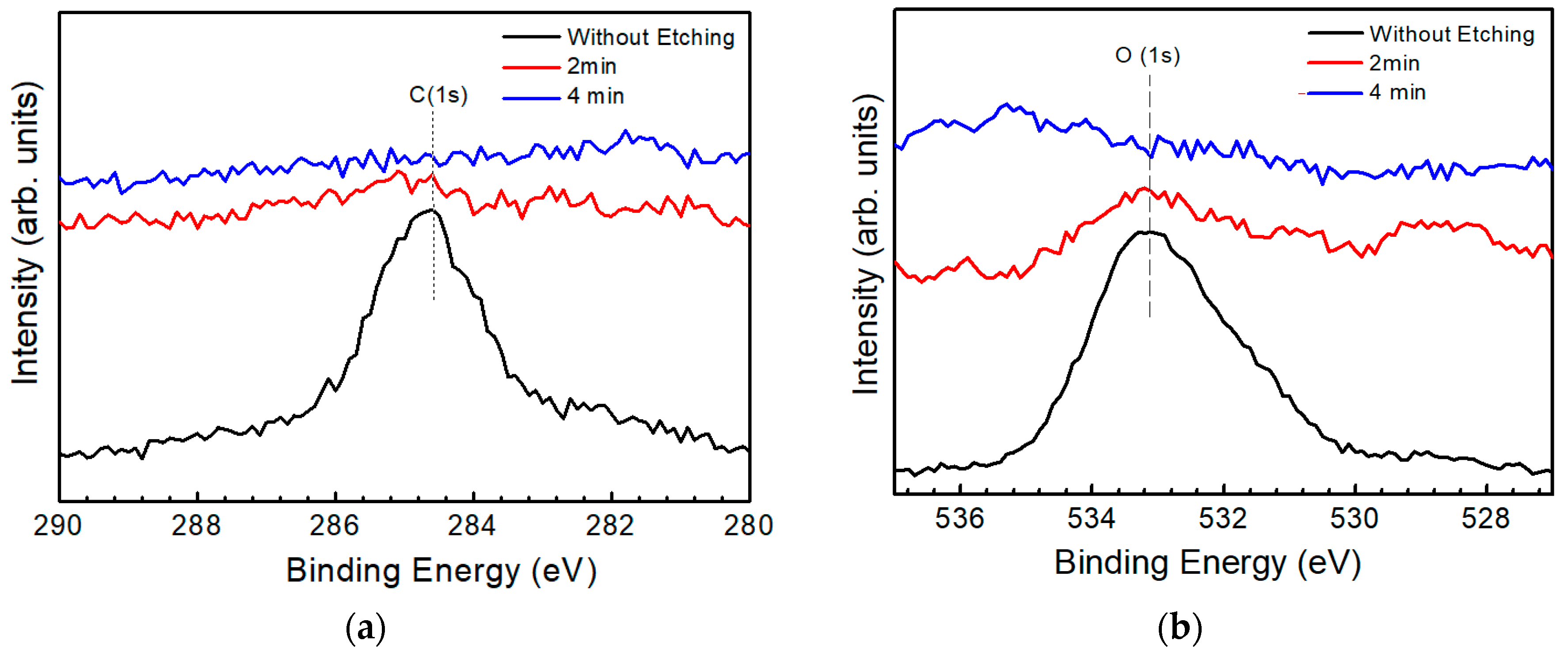
3.3. Identification of a Secondary Phase by XPS
 ), (
), (  ), (
), (  ) and (
) and (  ) symbols represent reference data of the Cu metal, Cu2S, CuS and CZTS film [19,20,21], respectively. The (
) symbols represent reference data of the Cu metal, Cu2S, CuS and CZTS film [19,20,21], respectively. The (  ) and (
) and (  ) symbols are experimental data of the surface and the bulk, respectively. The Auger parameter of Cu on the surface is quite different from that in the bulk. The parameter of Cu on the surface is near CuS in the CZTS film, while in the bulk it is near CZTS. The result indicates that the surface of the CZTS film is covered by a secondary phase of CuS and no such phase is observed in the CZTS bulk.
) symbols are experimental data of the surface and the bulk, respectively. The Auger parameter of Cu on the surface is quite different from that in the bulk. The parameter of Cu on the surface is near CuS in the CZTS film, while in the bulk it is near CZTS. The result indicates that the surface of the CZTS film is covered by a secondary phase of CuS and no such phase is observed in the CZTS bulk. ), (
), (  ) and (
) and (  ) symbols correspond to reference data of Zn metal, ZnS and CZTS film [25,26,27], respectively. The (
) symbols correspond to reference data of Zn metal, ZnS and CZTS film [25,26,27], respectively. The (  ) and (
) and (  ) symbols are experimental data of the surface and the bulk, respectively. Judging from the figure, the Auger parameter of Zn on the surface is nearly that of ZnS, while in the bulk it is nearly that of CZTS. The result indicates that the secondary phase of ZnS mainly exists on the surface and in the bulk, ZnS is not detected, which is consistent with a previous result.
) symbols are experimental data of the surface and the bulk, respectively. Judging from the figure, the Auger parameter of Zn on the surface is nearly that of ZnS, while in the bulk it is nearly that of CZTS. The result indicates that the secondary phase of ZnS mainly exists on the surface and in the bulk, ZnS is not detected, which is consistent with a previous result. ), (
), (  ), (
), (  ), (
), (  ) and (
) and (  ) symbols represent the reference data of Sn metal, SnS, Sn2S3 and CZTS in a CIGS film [25,29,31], respectively. The (
) symbols represent the reference data of Sn metal, SnS, Sn2S3 and CZTS in a CIGS film [25,29,31], respectively. The (  ) and (
) and (  ) symbols are the experimental data of the surface and the bulk, respectively. The Auger parameter of Sn on the surface is nearly that of the SnS compound, while in the bulk it is nearly that of CZTS, Sn2S3 and SnS2. According to the XRD result, the main structure of the film is CZTS, which shows that the bulk is CZTS but not an SnS compound. Thus, it can be concluded that the surface of the CZTS film is covered by a secondary phase of SnS but no such secondary phase is observed in the bulk, which accords with our previous result. Although it has also been reported that ternary compounds such as Cu2SnS3 and Cu3SnS4 may also exist on the surface [32], they were not identified in the XPS spectra in this work.
) symbols are the experimental data of the surface and the bulk, respectively. The Auger parameter of Sn on the surface is nearly that of the SnS compound, while in the bulk it is nearly that of CZTS, Sn2S3 and SnS2. According to the XRD result, the main structure of the film is CZTS, which shows that the bulk is CZTS but not an SnS compound. Thus, it can be concluded that the surface of the CZTS film is covered by a secondary phase of SnS but no such secondary phase is observed in the bulk, which accords with our previous result. Although it has also been reported that ternary compounds such as Cu2SnS3 and Cu3SnS4 may also exist on the surface [32], they were not identified in the XPS spectra in this work.4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.N.; Sung, S.J.; Son, D.H.; Kim, D.H.; Gansukh, M.; Cheong, H.; Kang, J.K. Solution-processed Cu2ZnSnS4 Absorbers Prepared by Appropriate Inclusion and Removal of Thiourea. RSC Adv. 2014, 4, 9118–9125. [Google Scholar] [CrossRef]
- Su, Z.H.; Tan, J.M.R.; Li, X.L.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation Substitution of Solution-Processed Cu2ZnSnS4 Thin Film Solar Cell with over 9% Efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Hadke, S.H.; Levcenko, S.; Lie, S.; Hages, C.J.; Márquez, J.A.; Unold, T.; Wong, L.H. Synergistic Effects of Double Cation Substitution in Solution-Processed CZTS Solar Cells with over 10%. Adv. Energy Mater. 2018, 8, 1802540. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Akhavan, V.A.; Goodfellow, B.W.; Panthani, M.G.; Steinhagen, C.; Harvey, T.B.; Stolle, C.J.; Korgel, B. Colloidal CIGS and CZTS Nanocrystals: A Precursor Route to Printed Photovoltaics. J. Solid State Chem. 2012, 189, 2–12. [Google Scholar] [CrossRef]
- Yoshida, S. Solar Frontier Achieves World Record Thin-Film Solar Cell Efficiency of 23.35%. Available online: http://www.solar-frontier.com/eng/news/2019/0117_press.html (accessed on 17 January 2019).
- Garcia-Llamas, E.; Merino, J.M.; Gunder, R.; Neldner, K.; Greiner, D.; Steigert, A.; Giraldo, S.; Izquierdo-Roca, V.; Saucedo, E.; León, M.; et al. Cu2ZnSnS4 Thin Film Solar Cells Grown by Fast Thermal Evaporation and Thermal Treatment. Sol. Energy 2017, 141, 236–241. [Google Scholar] [CrossRef]
- Kermadia, S.; Sali, S.; Zougar, L.; Boumaour, M.; Gunder, R.; Schorr, S.; Izquierdo-Roca, V.; Pérez-Rodríguez, A. An In-depth Investigation on the Grain Growth and the Formation of Secondary Phases of Ultrasonic-sprayed Cu2ZnSnS4 based Thin Films Assisted by Na Crystallization Catalyst. Sol. Energy 2018, 176, 277–286. [Google Scholar] [CrossRef]
- Babichuk, I.S.; Golovynskyi, S.; Brus, V.V.; Babichuk, I.V.; Datsenko, O.; Li, J.; Xu, G.W.; Golovynska, I.; Hreshchuk, O.M.; Orletskyi, I.G.; et al. Secondary Phases in Cu2ZnSnS4 Films Obtained by Spray Pyrolysis at different Substrate Temperatures and Cu Contents. Mater. Lett. 2018, 216, 173–175. [Google Scholar] [CrossRef]
- Just, J.; Lützenkirchen-Hecht, D.; Müller, O.; Frahm, R.; Unold, T. Depth Distribution of Secondary Phases in Kesterite Cu2ZnSnS4 by Angle-resolved X-ray Absorption Spectroscopy. APL Mater. 2017, 5, 126106. [Google Scholar] [CrossRef]
- Scragg, J.J.; Ericson, T.; Kubart, T.; Edoff, M.; Platzer-Björkman, C. Chemical Insights into the Instability of Cu2ZnSnS4 Films during Annealing. Chem. Mater. 2011, 23, 4625–4633. [Google Scholar] [CrossRef]
- Zhang, X.F.; Fu, E.G.; Wang, Y.H.; Zhang, C. Fabrication of Cu2ZnSnS4 (CZTS) Nanoparticle Inks for Growth of CZTS Films for Solar Cells. Nanomaterials 2019, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Roscher, H.H.; Labusch, R. Preparation and Properties of CuInSe2 Thin Films Produced by Selenization of Co-sputtered Cu-In films. Thin Solid Films 1994, 251, 116–120. [Google Scholar] [CrossRef]
- Khare, A.; Himmetoglu, B.; Cococcioni, M.; Aydil, E.S. First Principles Calculation of the Electronic Properties and Lattice Dynamics of Cu2ZnSn(S1−xSex)4. J. Appl. Phys. 2012, 111, 123704. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Rao, C.N.R. A Combined EELS-XPS Study of Molecularly Chemisorbed Oxygen on Silver Surfaces: Evidence for Superoxo and Peroxo Species. Surf. Sci. 1987, 186, L575–L580. [Google Scholar] [CrossRef]
- Awadallah, O.; Cheng, Z. Study of the Fundamental Phase Formation Mechanism of Sol-gel Sulfurized Cu2ZnSnS4 Thin Films using in situ Raman Spectroscopy. Sol. Energy Sol. Cell 2018, 176, 222–229. [Google Scholar] [CrossRef]
- Tao, J.H.; Liu, J.F.; Chen, L.L.; Cao, H.Y.; Meng, X.K.; Zhang, Y.B.; Zhang, C.J.; Sun, L.; Yang, P.X.; Chu, J.H. 7.1% Efficient Co-electroplated Cu2ZnSnS4 Thin Film Solar Cells with Sputtered CdS Buffer Layers. Green Chem. 2016, 18, 550–557. [Google Scholar] [CrossRef]
- Caporali, S.; Tolstogouzov, A.; Teodoro, O.M.N.D.; Innocenti, M.; Benedetto, F.D.; Cinotti, S.; Picca, R.A.; Sportelli, M.C.; Cioffi, N. Sn-deficiency in the Electrodeposited Ternary CuxSnySz Thin Films by ECALE. Sol. Energy Mater. Sol. Cell 2015, 138, 9–16. [Google Scholar] [CrossRef]
- Nakai, I.; Sugitani, Y.; Nagashima, K.; Niwa, Y. X-ray Photoelectron Spectroscopic Study of Copper Minerals. J. Inorg. Nucl. Chem. 1978, 40, 789–791. [Google Scholar] [CrossRef]
- Cabrera-German, D.; García-Valenzuela, J.A.; Martínez-Gil, M.; Suárez-Campos, G.; Montiel-González, Z.; Sotelo-Lerma, M.; Cota-Leal, M. Assessing the chemical state of chemically deposited copper sulfide: A quantitative analysis of the X-ray photoelectron spectra of the amorphous-to-covellite transition phases. Appl. Surf. Sci. 2019, 481, 281–295. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced Analysis of Copper X-ray Photoelectron Spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Tao, J.H.; Chen, L.L.; Cao, H.Y.; Zhang, C.J.; Liu, J.F.; Zhang, Y.B.; Huang, L.; Jiang, J.C.; Yang, P.X.; Chu, J.H. Co-electrodeposited Cu2ZnSnS4 Thin-Film Solar Cells with over 7% Efficiency Fabricated via Fine-tuning of the Zn Content in Absorber Layers. J. Mater. Chem. A 2016, 4, 3798–3805. [Google Scholar] [CrossRef]
- Ge, S.J.; Gao, H.; Hong, R.J.; Li, J.J.; Mai, Y.H.; Lin, X.Z.; Yang, G.W. Improvement of Cu2ZnSn(S,Se)4 Solar Cells by Adding N,N-Dimethylformamide to the Dimethyl Sulfoxide-Based Precursor Ink. ChemSusChem 2019, 12, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Winiarski, J.; Tylus, W.; Szczygie, B. EIS and XPS Investigations on the Corrosion Mechanism of Ternary Zn-Co-Mo Alloy Coatings in NaCl Solution. Appl. Surf. Sci. 2016, 364, 455–466. [Google Scholar] [CrossRef]
- Powell, C.J. Recommended Auger Parameters for 42 Elemental Solids. J. Electron Spectrosc. 2012, 185, 1–3. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray Photoelectron Spectroscopy Analysis of Copper and Zinc Oxides and Sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Langer, D.W.; Vesely, C.J. Electronic Core Levels of Zinc Chalcogenides. Phys. Rev. B 1970, 2, 4885–4892. [Google Scholar] [CrossRef]
- Lin, A.W.C.; Armstrong, N.R.; Kuwana, T. X-ray Photoelectron/Auger Electron Spectroscopic Studies of Tin and Indium Metal Foils and Oxides. Anal. Chem. 1977, 49, 1228–1235. [Google Scholar] [CrossRef]
- Cruz, M.; Morales, J.; Espinos, J.P.; Sanz, J. XRD, XPS and Sn NMR Study of Tin Sulfides Obtained by Using Chemical Vapor Transport Methods. J. Solid State Chem. 2003, 175, 359–365. [Google Scholar] [CrossRef]
- Kövér, L.; Moretti, G.; Kovács, Z.; Sanjinés, R.; Cserny, I.; Margaritondo, G.; Pálinkás, J.; Adachi, H. High Resolution Photoemission and Auger Parameter Studies of Electronic Structure of Tin Oxides. J. Vac. Sci. Technol. A 1995, 13, 1382–1388. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, I.Y.; Surywanshi, M.P.; Ghorpade, U.V.; Lee, D.S.; Kim, J.H. Fabrication of Cu2SnS3 Thin Film Solar Cells using Cu/Sn Layered Metallic Precursors Prepared by a Sputtering Process. Sol. Energy. 2017, 145, 27–32. [Google Scholar] [CrossRef]
- Fontané, X.; Calvo-Barrio, L.; Izquierdo-Roca, V.; Saucedo, E.; Pérez-Rodriguez, A.; Morante, J.R.; Berg, D.M.; Dale, P.J.; Siebentritt, S. In-depth Resolved Raman Scattering Analysis for the Identification of Secondary Phases: Characterization of Cu2ZnSnS4 Layers for Solar Cell Applications. Appl. Phys. Lett. 2011, 98, 181905. [Google Scholar] [CrossRef]
- Ramanathan, K.; Contreras, M.A.; Perkins, C.L.; Asher, S.; Hasoon, F.S.; Keane, J.; Young, D.; Romero, M.; Metzger, W.; Noufi, R.; et al. Properties of 19.2% Efficiency ZnO/CdS/CuInGaSe2 Thin-Film Solar Cells. Prog. Photovolt. 2003, 11, 225–230. [Google Scholar] [CrossRef]
- Abous-Ras, D.; Kirchartz, T.; Rau, U. Advanced Characterization Techniques for Thin Film Solar Cells, 1st ed.; WILEY-VCH Verlag GmbH&Co. KGaA: Weinheim, Germany, 2011; p. 6. [Google Scholar]
- Christians, J.A.; Manser, J.S.; Kamat, P.V. Best Practices in Perovskite Solar Cell Efficiency Measurements. Avoiding the Error of Making Bad Cells Look Good. J. Phys. Chem. Lett. 2015, 6, 852–857. [Google Scholar] [CrossRef] [PubMed]



 ), (
), (  ), (
), (  ) and (
) and (  ) symbols show the reference data of Cu metal, Cu2S, CuS and CZTS in the CZTS film, respectively. The (
) symbols show the reference data of Cu metal, Cu2S, CuS and CZTS in the CZTS film, respectively. The (  ) symbol shows the experimental data on the surface in this work. (
) symbol shows the experimental data on the surface in this work. (  ) shows the experimental data of the CZTS in the bulk.
) shows the experimental data of the CZTS in the bulk.
 ), (
), (  ), (
), (  ) and (
) and (  ) symbols show the reference data of Cu metal, Cu2S, CuS and CZTS in the CZTS film, respectively. The (
) symbols show the reference data of Cu metal, Cu2S, CuS and CZTS in the CZTS film, respectively. The (  ) symbol shows the experimental data on the surface in this work. (
) symbol shows the experimental data on the surface in this work. (  ) shows the experimental data of the CZTS in the bulk.
) shows the experimental data of the CZTS in the bulk.

 ), (
), (  ) and (
) and (  ) symbols show reference data of Zn metal, ZnS and CZTS in CZTS film, respectively. The (
) symbols show reference data of Zn metal, ZnS and CZTS in CZTS film, respectively. The (  ) symbol shows experimental data on the surface in this work. (
) symbol shows experimental data on the surface in this work. (  ) shows experimental data in the bulk of a CZTS film.
) shows experimental data in the bulk of a CZTS film.
 ), (
), (  ) and (
) and (  ) symbols show reference data of Zn metal, ZnS and CZTS in CZTS film, respectively. The (
) symbols show reference data of Zn metal, ZnS and CZTS in CZTS film, respectively. The (  ) symbol shows experimental data on the surface in this work. (
) symbol shows experimental data on the surface in this work. (  ) shows experimental data in the bulk of a CZTS film.
) shows experimental data in the bulk of a CZTS film.

 ), (
), (  ), (
), (  ) (
) (  ) and (
) and (  ) symbols show the reference data of Sn metal, SnS, Sn2S3, SnS2 and CZTS in a CZTS film, respectively. The (
) symbols show the reference data of Sn metal, SnS, Sn2S3, SnS2 and CZTS in a CZTS film, respectively. The (  ) symbol shows experimental data on the surface in this work. (
) symbol shows experimental data on the surface in this work. (  ) shows experimental data in the bulk of a CZTS film.
) shows experimental data in the bulk of a CZTS film.
 ), (
), (  ), (
), (  ) (
) (  ) and (
) and (  ) symbols show the reference data of Sn metal, SnS, Sn2S3, SnS2 and CZTS in a CZTS film, respectively. The (
) symbols show the reference data of Sn metal, SnS, Sn2S3, SnS2 and CZTS in a CZTS film, respectively. The (  ) symbol shows experimental data on the surface in this work. (
) symbol shows experimental data on the surface in this work. (  ) shows experimental data in the bulk of a CZTS film.
) shows experimental data in the bulk of a CZTS film.

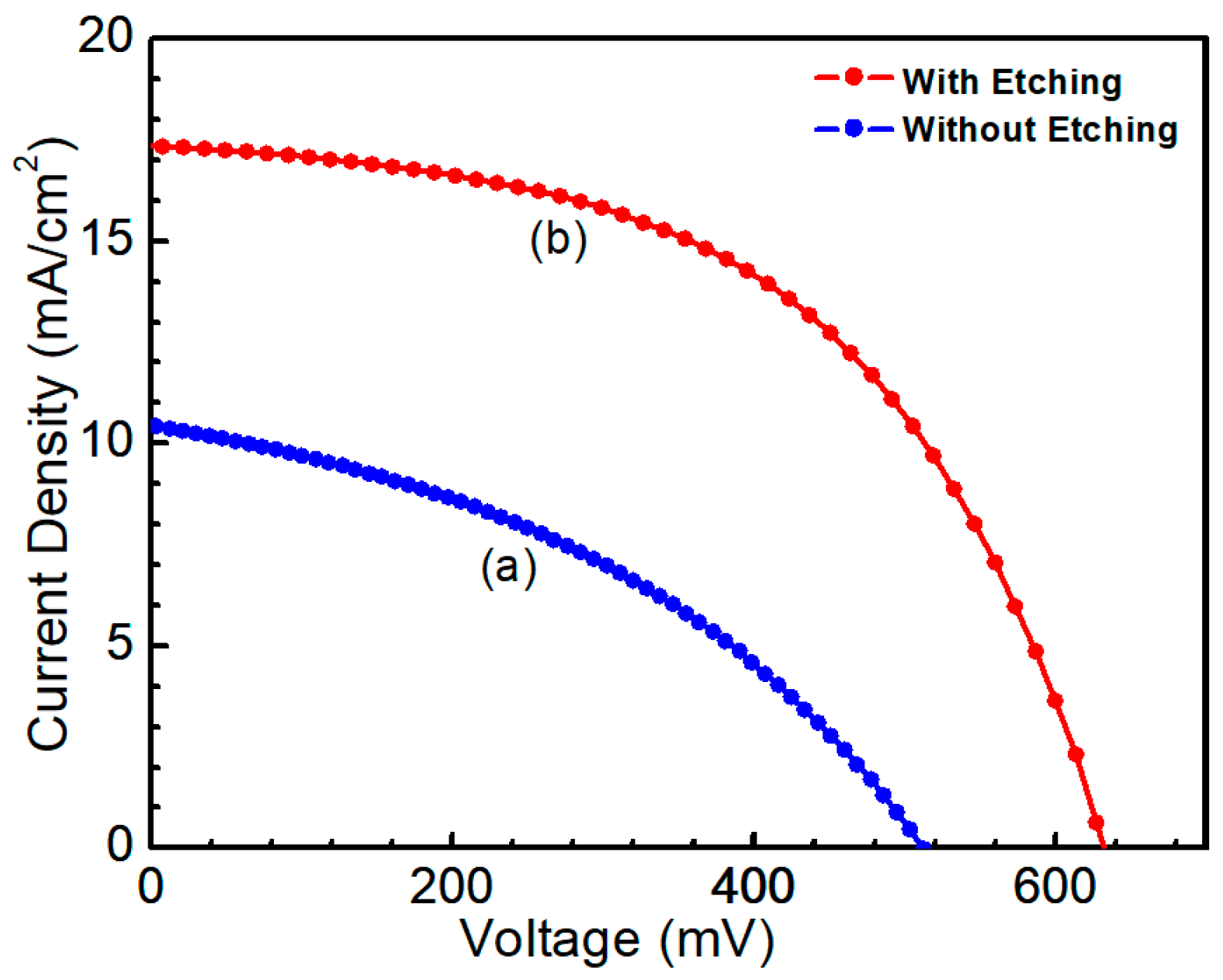
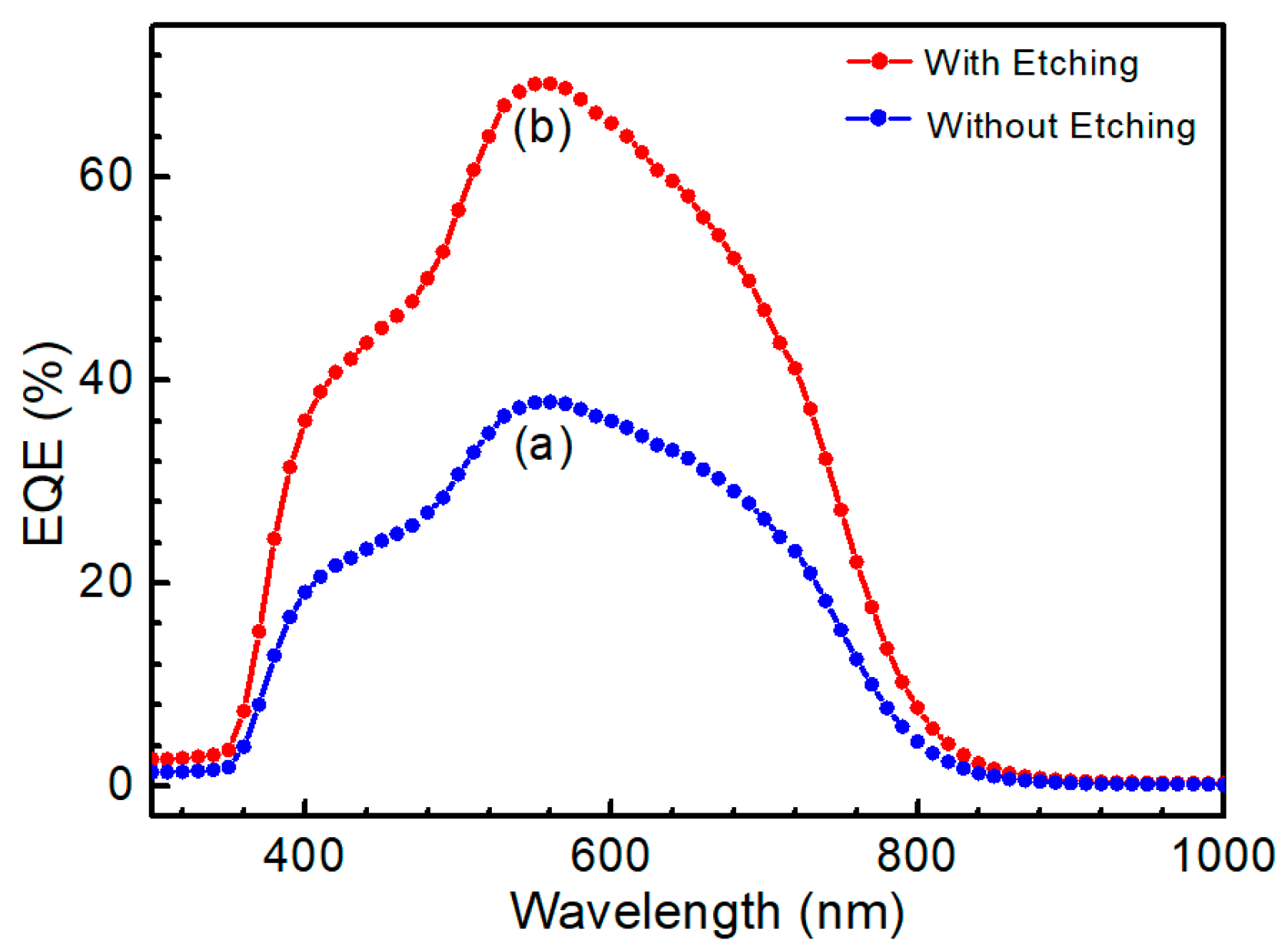
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wu, H.; Fu, E.; Wang, Y. In-Depth Characterization of Secondary Phases in Cu2ZnSnS4 Film and Its Application to Solar Cells. Nanomaterials 2019, 9, 855. https://doi.org/10.3390/nano9060855
Zhang X, Wu H, Fu E, Wang Y. In-Depth Characterization of Secondary Phases in Cu2ZnSnS4 Film and Its Application to Solar Cells. Nanomaterials. 2019; 9(6):855. https://doi.org/10.3390/nano9060855
Chicago/Turabian StyleZhang, Xianfeng, Hongde Wu, Engang Fu, and Yuehui Wang. 2019. "In-Depth Characterization of Secondary Phases in Cu2ZnSnS4 Film and Its Application to Solar Cells" Nanomaterials 9, no. 6: 855. https://doi.org/10.3390/nano9060855
APA StyleZhang, X., Wu, H., Fu, E., & Wang, Y. (2019). In-Depth Characterization of Secondary Phases in Cu2ZnSnS4 Film and Its Application to Solar Cells. Nanomaterials, 9(6), 855. https://doi.org/10.3390/nano9060855






