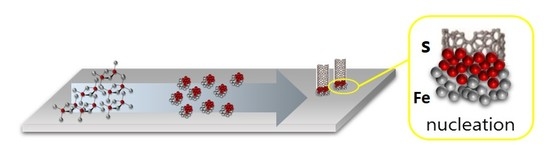
Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CNT Forest
2.2. Characterization
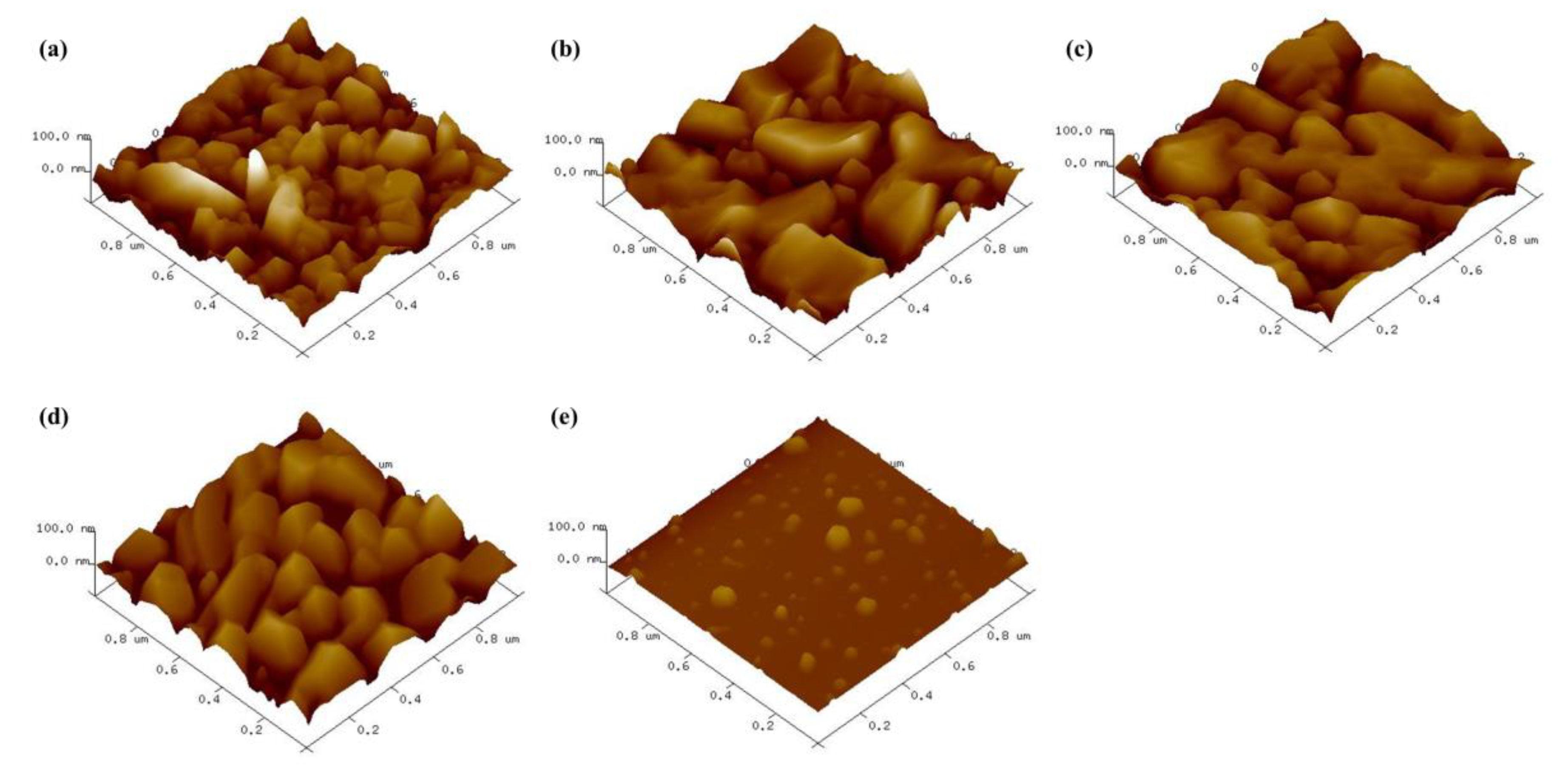
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Fan, S. Spinning continuous carbon nanotube yarns. Nature 2002, 419, 801. [Google Scholar] [CrossRef]
- Ogasawara, T.; Tsuda, T.; Takeda, N. Stress–strain behavior of multi-walled carbon nanotube/PEEK composites. Compos. Sci. Technol. 2011, 71, 73–78. [Google Scholar] [CrossRef]
- Wardle, B.L.; Saito, D.S.; Garcia, E.J.; Hart, A.J.; Villoria, R.G.D.; Verploegen, E.A. Fabrication and Characterization of Ultrahigh-Volume- Fraction Aligned Carbon Nanotube–Polymer Composites. Adv. Mater. 2008, 20, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Moon, S.Y.; Inoue, Y.; Shimamura, Y. Mechanical properties of aligned multi-walled carbon nanotube/epoxy composites processed using a hot-melt prepreg method. Compos. Sci. Technol. 2011, 71, 1826–1833. [Google Scholar] [CrossRef]
- Tsuda, T.; Ogasawara, T.; Moon, S.Y.; Nakamoto, K.; Takeda, N.; Shimamura, Y.; Inoue, Y. Stress transfer efficiency in aligned multi-wall carbon nanotubes sheet/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2014, 67, 16–21. [Google Scholar] [CrossRef]
- Tsuda, T.; Ogasawara, T.; Moon, S.Y.; Nakamoto, K.; Takeda, N.; Shimamura, Y.; Inoue, Y. Nanoscopic observations for evaluating the failure process of aligned multi-walled carbon nanotube/epoxy composites. Compos. Sci. Technol. 2013, 88, 48–56. [Google Scholar] [CrossRef]
- Lepró, X.; Lima, M.D.; Baughman, R.H. Spinnable carbon nanotube forests grown on thin, flexible metallic substrates. Carbon 2010, 48, 3621–3627. [Google Scholar] [CrossRef]
- Tran, C.D.; Humphries, W.; Smith, S.M.; Huynh, C.; Lucas, S. Improving the tensile strength of carbon nanotube spun yarns using a modified spinning process. Carbon 2009, 47, 2662–2670. [Google Scholar] [CrossRef]
- Huynh, C.P.; Hawkins, S.C.; Gengenbach, T.R.; Hymphries, W.; Clenn, M.; Simon, G.P. Evolution of directly-spinnable carbon nanotube catalyst structure by recycling analysis. Carbon 2013, 62, 204–212. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.P.; Kim, H.R.; Lee, J.G.; Hong, K.H. Synthesis of high-quality carbon nanotube fibers by controlling the effects of sulfur on the catalyst agglomeration during the direct spinning process. RSC Adv. 2015, 5, 41894–41900. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Ren, F.; Wang, Q.; Pfefferle, L.D.; Haller, G.L.; Chen, Y. Chiral-Selective CoSO4/SiO2 Catalyst for (9,8) Single-Walled Carbon Nanotube Growth. ACS Nano 2013, 7, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, F.; Liu, C.; Si, R.; Yu, D.; Pfefferle, L.D.; Haller, G.L.; Chen, Y. CoSO4/SiO2 catalyst for selective synthesis of (9, 8) single-walled carbon nanotubes: Effect of catalyst calcination. J. Catal. 2013, 300, 91–101. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Sui, X.; Karhan, H.E.; Wang, X.; Chen, Y. Selective synthesis of single walled carbon nanotubes on metal (iron, nickel or cobalt) sulfate-based catalysts. Carbon 2018, 129, 128–136. [Google Scholar] [CrossRef]
- Zhong, G.; Warner, J.H.; Fouquet, M.; Robertson, A.W.; Chen, B.; Robertson, J. Growth of ultrahigh density single walled carbon Nanotube Forests by improved catalyst design. ACS Nano 2012, 6, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.F.; Iwasaki, T.; Kawarada, H. Semi-quantitative study on fabrication of densely packed and vertically aligned singl-walled carbon nanotuebs. Carbon 2006, 44, 2009–2014. [Google Scholar] [CrossRef]
- Zhong, G.; Xie, R.; Yang, J.; Robertson, J. Single-step CVD growth of high-density carbon nanotube forests on metallic Ti coatings through catalyst engineering. Carbon 2014, 67, 680–687. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Xie, R.; Fouquet, M.; Cartwright, R.; Hardeman, D.; Yang, J.; Robertson, J. Measurement of area density of vertically aligned carbon nanotube forests by the weight-gain method. J. Appl. Phys. 2013, 113, 144309. [Google Scholar] [CrossRef]
- Pumera, M.; Iwai, H. Multicomponent Metallic Impurities and Their Influence upon the Electrochemistry of Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 4401–4405. [Google Scholar] [CrossRef]
- Sanchez, S.; Fabregas, E.; Pumera, M. Electrochemical activation of carbon nanotube/polymer composites. Phys. Chem. Chem. Phys. 2009, 11, 182–186. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R.; Lieber, C.M.; Hunter, M.; McClure, T.; Dresselhaus, G.; Dresselhaus, M.S. Structural (n,m) Determination of Isolated Single-Wall Carbon Nanotubes by Resonant Raman Scattering. Phys. Rev. Lett. 2001, 86, 1118. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Huh, Y.; Lee, J.Y. Temperature effect on the growth of carbon nanotubes using thermal chemical vapor deposition. Chem. Phys. Lett. 2001, 343, 33–38. [Google Scholar] [CrossRef]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide metals. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Hou, P.X.; Xu, S.T.; Ying, Z.; Yang, Q.H.; Liu, C.; Cheng, H.M. Hydrogen adsorption/desorption behavior of multi-walled carbon nanotubes with different diameters. Carbon 2003, 41, 2471–2476. [Google Scholar] [CrossRef]
- Moon, S.Y. A spectroscopic study on surface and structural transitions of carbon nanotubes under different atmospheres. RSC Adv. 2016, 6, 79401–79404. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.Y.; Kang, I.J.; Kim, S.M.; Kim, W.S. Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests. Nanomaterials 2019, 9, 889. https://doi.org/10.3390/nano9060889
Moon SY, Kang IJ, Kim SM, Kim WS. Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests. Nanomaterials. 2019; 9(6):889. https://doi.org/10.3390/nano9060889
Chicago/Turabian StyleMoon, Sook Young, In Ji Kang, Seung Min Kim, and Woo Sik Kim. 2019. "Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests" Nanomaterials 9, no. 6: 889. https://doi.org/10.3390/nano9060889
APA StyleMoon, S. Y., Kang, I. J., Kim, S. M., & Kim, W. S. (2019). Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests. Nanomaterials, 9(6), 889. https://doi.org/10.3390/nano9060889