Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance
Abstract
:1. Introduction
2. Experiment
2.1. Growth of ZnO NRs
2.2. Preparation of NiO/ZnO Heterostructures
3. Results and Discussion
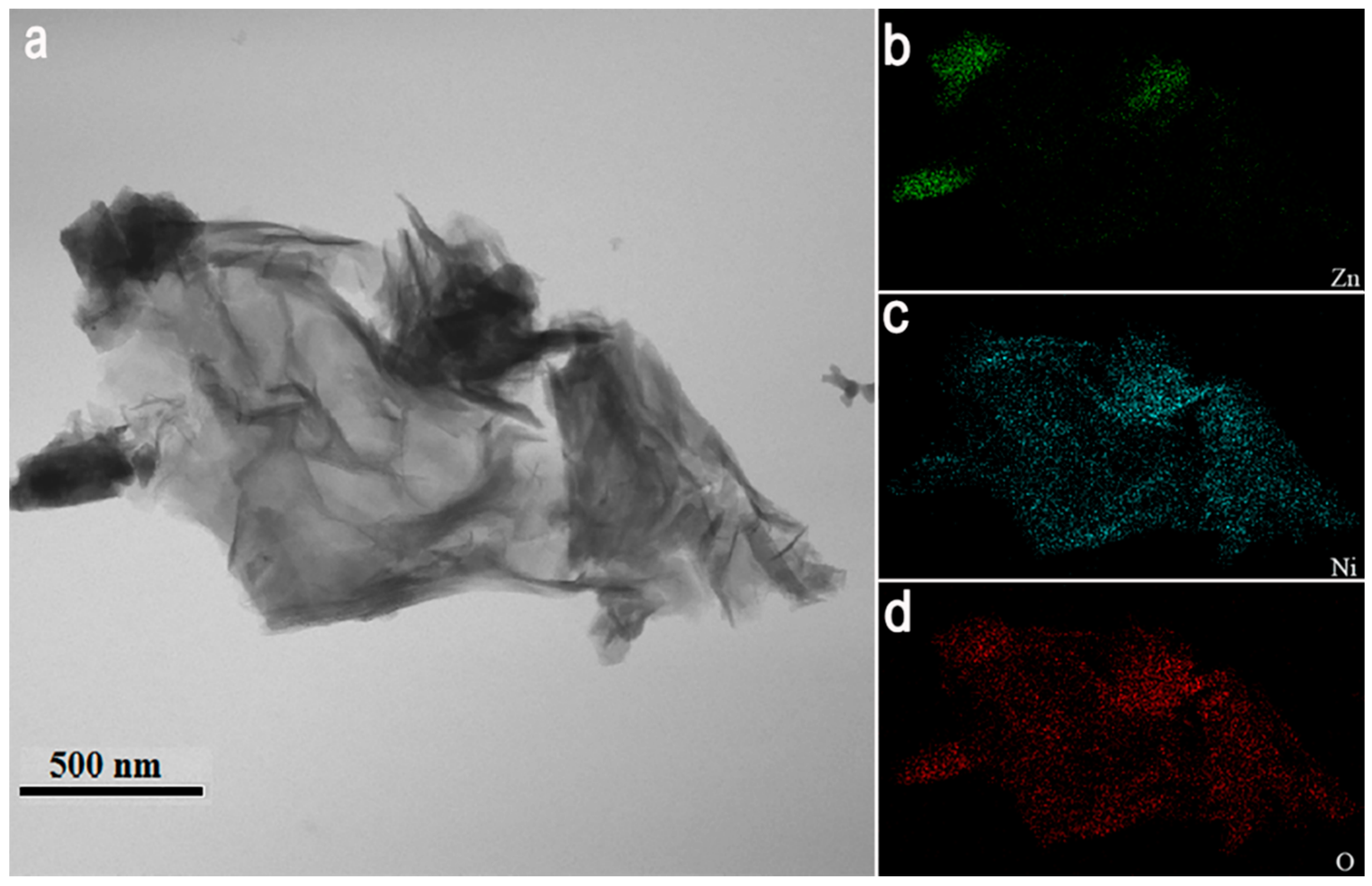
3.1. Characterizations of Pure ZnO Nanorods and NiO/ZnO Heterostructures
3.2. Gas Sensing Properties
3.3. Sensing Mechanism of NiO/ZnO-Heterostructures-Based Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, F.Y.; Cao, X.Q.; Qu, F.L.; Asiri, A.M.; Sun, X.P. Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sens. Actuators B Chem. 2018, 255, 1254–1261. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Li, Z.J.; Niu, X.Y.; Lin, Z.J.; Wang, N.N.; Shen, H.H.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z.G. Hydrothermally synthesized CeO2 nanowires for H2S sensing at room temperature. J. Alloys Compd. 2016, 682, 647–653. [Google Scholar] [CrossRef]
- Kim, J.; Yong, K. Mechanism Study of ZnO Nanorod-Bundle Sensors for H2S Gas Sensing. J. Phys. Chem. C 2011, 115, 7218–7224. [Google Scholar] [CrossRef]
- Tian, H.L.; Fan, H.Q.; Li, M.M.; Ma, L.T. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Na, C.W.; Park, S.Y.; Lee, J.H. Punched ZnO nanobelt networks for highly sensitive gas sensors. Sens. Actuators B Chem. 2012, 174, 495–499. [Google Scholar] [CrossRef]
- Mane, A.A.; Moholkar, A.V. Orthorhombic MoO3 nanobelts based NO2 gas sensor. Appl. Surf. Sci. 2017, 405, 427–440. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhu, C.L.; Xiao, G. Highly conductive titanium oxide nanotubes chemical sensors. Nanoscale 2015, 208, 165–170. [Google Scholar]
- Tam, K.H.; Cheung, C.K.; Leung, Y.H.; Djurisic, A.B.; Luig, C.C.; Beling, C.D.; Fung, S.; Kwok, W.M.; Chan, W.K.; Philips, D.L.; et al. Defects in ZnO nanorods prepared by hydrothermal method. J. Phys. Chem. B 2006, 110, 20865–20871. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Nguyen, N.T.; Walton, A.J.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E.; et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.L.; Li, Z.J.; Ma, J.Y.; Wang, L.; Yang, J.; Du, B.; Yu, Q.K.; Zu, X.T. Highly sensitive surface acoustic wave (SAW) humidity sensors based on sol-gel SiO2 films: Investigations on the sensing property and mechanism. Sens. Actuators B Chem. 2015, 215, 283–291. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, G.Z.; Mi, R.D.; Deng, C.K.; Gao, P.Z. An environment-benign method for the synthesis of p-NiO/n-ZnO heterostructure with excellent performance for gas sensing and photocatalysis. Sens. Actuators B Chem. 2014, 191, 537–544. [Google Scholar] [CrossRef]
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Guo, J.; Zhang, J.; Girot, E.; Mozet, K.; Medjahdi, G.; Schneider, R. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO PN heterojunction. Sens. Actuators B Chem. 2014, 200, 288–296. [Google Scholar] [CrossRef]
- Akram, M.A.; Javed, S.; Islam, M.; Mujahid, M.; Safdar, A. Arrays of CZTS sensitized ZnO/ZnS and ZnO/ZnSe core/shell nanorods for liquid junction nanowire solar cells. Sol. Energy Mater. Sol. Cells 2016, 146, 121–128. [Google Scholar] [CrossRef]
- Ginting, R.T.; Lee, H.B.; Tan, S.T.; Tan, C.H.; Jumali, M.H.H.; Yap, C.C.; Kang, J.W.; Yahaya, M. A Simple Approach Low-Temperature Solution Process for Preparation of Bismuth-Doped ZnO Nanorods and Its Application in Hybrid Solar Cells. J. Phys. Chem. C 2016, 120, 771–780. [Google Scholar] [CrossRef]
- Dang, V.Q.; Trung, T.Q.; Duy, L.T.; Kim, B.Y.; Siddiqui, S.; Lee, W.; Lee, N.E. High-Performance Flexible Ultraviolet (UV) Phototransistor Using Hybrid Channel of Vertical ZnO Nanorods and Graphene. ACS Appl. Mater. Interfaces 2015, 7, 11032–11040. [Google Scholar] [CrossRef]
- Dai, W.; Pan, X.H.; Chen, S.S.; Chen, C.; Wen, Z.; Zhang, H.H.; Ye, Z.Z. Honeycomb-like NiO/ZnO heterostructured nanorods: Photochemical synthesis, characterization, and enhanced UV detection performance. J. Mater. Chem. C 2014, 2, 4606–4614. [Google Scholar] [CrossRef]
- Su, X.F.; Chen, J.B.; He, R.M.; Li, Y.; Wang, J.; Wang, C.W. The preparation of oxygen-deficient ZnO nanorod arrays and their enhanced field emission. Mater. Sci. Semicond. Process. 2017, 67, 55–61. [Google Scholar] [CrossRef]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Tak, Y.; Park, D.; Yong, K. Characterization of ZnO nanorod arrays fabricated on Si wafers using a low-temperature synthesis method. J. Vac. Sci. Technol. B 2006, 24, 2047–2052. [Google Scholar] [CrossRef]
- Zhou, F.; Jing, W.X.; Xu, Y.X.; Chen, Z.; Jiang, Z.D.; Wei, Z.Y. Performance enhancement of ZnO nanorod-based enzymatic glucose sensor via reduced graphene oxide deposition and UV irradiation. Sens. Actuators B Chem. 2019, 208, 377–385. [Google Scholar] [CrossRef]
- Hosseinia, Z.S.; Irajizadbc, A.; Mortezaalia, A. Room temperature H2S gas sensor based on rather aligned ZnO nanorods with flower-like structures. Sens. Actuators B Chem. 2015, 207, 865–871. [Google Scholar] [CrossRef]
- Fu, D.Y.; Zhu, C.L.; Zhang, X.T.; Li, C.Y.; Chen, Y.J. Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A 2016, 4, 1390–1398. [Google Scholar] [CrossRef]
- Yu, T.T.; Cheng, X.L.; Zhang, X.F.; Sui, L.L.; Xu, Y.M.; Gao, S.; Zhao, H.; Huo, L. Highly sensitive H2S detection sensors at low temperature based on hierarchically structured NiO porous nanowall arrays. J. Mater. Chem. A 2015, 3, 11991–11999. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Sharma, P.K.; Datta, N.; Kaur, M.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Room temperature H2S sensor based on Au modified ZnO nanowires. Sens. Actuators B Chem. 2013, 186, 718–726. [Google Scholar] [CrossRef]
- Li, Z.J.; Lin, Z.J.; Wang, N.N.; Huang, Y.W.; Wang, J.Q.; Liu, W.; Fu, Y.Q.; Wang, Z.G. Facile synthesis of alpha-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater. Des. 2016, 110, 532–539. [Google Scholar] [CrossRef]
- Li, Z.J.; Huang, Y.W.; Zhang, S.C.; Chen, W.M.; Kuang, Z.; Ao, D.Y.; Liu, W.; Fu, Y.Q. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar]
- Li, Z.J.; Wang, J.Q.; Wang, N.N.; Yan, S.N.; Liu, W.; Fu, Y.Q.; Wang, Z.G. Hydrothermal synthesis of hierarchically flower-like CuO nanostructures with porous nanosheets for excellent H2S sensing. J. Alloys Compd. 2017, 725, 1136–1143. [Google Scholar] [CrossRef]
- Li, D.J.; Zu, X.T.; Ao, D.Y.; Tang, Q.B.; Fu, Y.Q.; Guo, Y.J.; Bilawal, K.; Faheem, M.B.; Li, L.; Li, S.; et al. High humidity enhanced surface acoustic wave (SAW) H2S sensors based on sol–gel CuO films. Sens. Actuators B Chem. 2019, 294, 55–61. [Google Scholar] [CrossRef]
- Li, D.J.; Tang, Y.L.; Ao, D.Y.; Xiang, X.; Wang, S.Y.; Zu, X.T. Ultra-highly sensitive and selective H2S gas sensor based on CuO with sub-ppb detection limit. Int. J. Hydrogen Energ. 2019, 44, 3985–3992. [Google Scholar] [CrossRef]
- Zhao, M.G.; Wang, X.C.; Ning, L.L.; Jia, J.F. Electrospun Cu-doped ZnO nanofibers for H2S sensing. Sens. Actuators B Chem. 2011, 156, 588–592. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.; Yong, K. CuO/ZnO Heterostructured Nanorods: Photochemical Synthesis and the Mechanism of H2S Gas Sensing. J. Phys. Chem. C 2012, 116, 15682–15691. [Google Scholar] [CrossRef]
- Tang, Y.L.; Li, Z.J.; Ma, J.Y.; Su, H.Q.; Guo, Y.J.; Wang, L.; Du, B.; Chen, J.J.; Zhou, W.L.; Yu, Q.K.; et al. Highly sensitive room-temperature surface acoustic wave (SAW) ammonia sensors based on Co3O4/SiO2 composite films. J. Hazard. Mater. 2014, 280, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Ma, J.Y.; Li, Z.J.; Su, H.Q.; Alkurd, N.R.; Zhou, W.L.; Wang, L.; Du, B.; Tang, Y.L.; Ao, D.Y.; et al. Surface acoustic wave ammonia sensor based on ZnO/SiO2 composite film. J. Hazard. Mater. 2015, 285, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Lin, J.C.; Ciou, Y.J.; Hwang, Y.R. Fabrication of a Novel Microsensor Consisting of Electrodeposited ZnO Nanorod-Coated Crossed Cu Micropillars and the Effects of Nanorod Coating Morphology on the Gas Sensing. ACS Appl. Mater. Interfaces 2014, 6, 11424–11438. [Google Scholar] [CrossRef] [PubMed]
- Hoa, L.T.; Tien, H.N.; Hur, S.H. A highly sensitive UV sensor composed of 2D NiO nanosheets and 1D ZnO nanorods fabricated by a hydrothermal process. Sens. Actuators A Phys. 2014, 207, 20–24. [Google Scholar] [CrossRef]
- Evingur, G.A.; Pekcan, O. Optical energy band gap of PAAm-GO composites. Compos. Struct. 2018, 183, 212–215. [Google Scholar] [CrossRef]
- Wang, R.C.; Chen, M.G. Enhanced photosensing and tunable luminescence from ZnO/NiO and ZnO/Ni core–shell nanorods. Sens. Actuators B Chem. 2013, 178, 212–216. [Google Scholar] [CrossRef]
- Wang, L.W.; Kang, Y.F.; Wang, Y.; Zhu, B.L.; Zhang, S.M.; Huang, W.P.; Wang, S.R. CuO nanoparticle decorated ZnO nanorod sensor for low-temperature H2S detection. Mater. Sci. Eng. C 2012, 32, 2079–2085. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.W.; Jeong, H.M.; Hwang, S.J.; Kwak, C.H.; Lee, J.H. Design of highly sensitive and selective Au@NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Qu, F.D.; Liu, J.; Wang, Y.; Zhou, J.R. Enhanced H2S sensing characteristics of CuO-NiO core-shell microspheres sensors. Sens. Actuators B Chem. 2015, 209, 515–523. [Google Scholar] [CrossRef]
- Jin, C.; Kim, H.; An, S.; Lee, C. Highly sensitive H2S gas sensors based on CuO-coated ZnSnO3 nanorods synthesized by thermal evaporation. Ceram. Int. 2012, 38, 5973–5978. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Does Crystal Shape Matter? Small 2010, 6, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice. Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Kim, H.R.; Choi, K.I.; Kim, K.M.; Kim, I.D.; Cao, G.; Lee, J.H. Ultra-fast responding and recovering C2H5OH sensors using SnO2 hollow spheres prepared and activated by Ni templates. Chem. Commun. 2010, 46, 5061–5063. [Google Scholar] [CrossRef]
- Kohl, D. Function and applications of gas sensor. J. Phys. D Appl. Phys. 2001, 34, R125–R149. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.M.; Chen, W. Fabrication of SnO-SnO2 nanocomposites with p-n heterojunctions for low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef]
- Geng, B.Y.; Wang, G.Z.; Jiang, Z.; Xie, T.; Sun, S.H.; Meng, G.W.; Zhang, L.D. Synthesis and optical properties of S-doped ZnO nanowires. Appl. Phys. Lett. 2003, 82, 4791. [Google Scholar] [CrossRef]










| Material | Detection Limit (ppm) | Gas Conc. (ppm) | T (°C) | Response (Ra/Rg or Rg/Ra) | Reference |
|---|---|---|---|---|---|
| ZnO Nanorods | - | 100 | 100 | 23 | [26] |
| CuO-ZnO Nanorods | - | 100 | 100 | 39 | [41] |
| NiO Thin film | 1 | 50 | 92 | 20.6 | [25] |
| Au-NiO Yolk-shell | 1.25 | 5 | 400 | 44.2 | [42] |
| CuO-NiO Microspheres | 10 | 100 | 260 | 47.6 | [43] |
| CuO-ZnSnO3 Nanowires | 25 | 100 | 100 | 2.2 | [44] |
| NiO/ZnO Heterostructures | 0.1 | 20 | 160 | 21.3 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, D.; Li, Z.; Fu, Y.; Tang, Y.; Yan, S.; Zu, X. Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance. Nanomaterials 2019, 9, 900. https://doi.org/10.3390/nano9060900
Ao D, Li Z, Fu Y, Tang Y, Yan S, Zu X. Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance. Nanomaterials. 2019; 9(6):900. https://doi.org/10.3390/nano9060900
Chicago/Turabian StyleAo, Dongyi, Zhijie Li, Yongqing Fu, Yongliang Tang, Shengnan Yan, and Xiaotao Zu. 2019. "Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance" Nanomaterials 9, no. 6: 900. https://doi.org/10.3390/nano9060900
APA StyleAo, D., Li, Z., Fu, Y., Tang, Y., Yan, S., & Zu, X. (2019). Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance. Nanomaterials, 9(6), 900. https://doi.org/10.3390/nano9060900







