Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
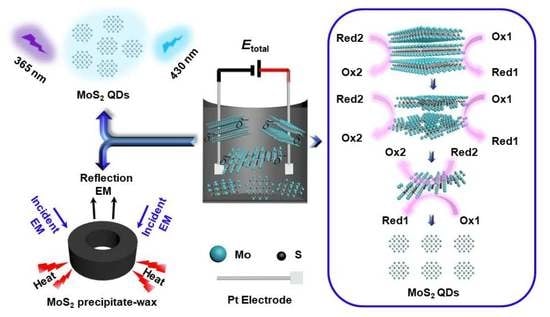
3.1. Preparation and Characterization of the MoS2 QDs and Precipitate Containing the Nanosheets
3.2. Applications of the As-Obtained MoS2 QDs
3.3. Application of Precipitate Containing the Nanosheets in EMW Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pavlović, S.; Peeters, F.M. Electronic properties of triangular and hexagonal MoS2 quantum dots. Phys. Rev. B 2015, 91, 155410. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Jiang, K.; Wang, C.; Zhang, C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 252, 183–190. [Google Scholar] [CrossRef]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.; Chang, M.T.; Lin, C.T.; Chang, K.D.; Yu, Y.C.; Wang, J.T.W.; Chang, C.S.; Li, L.J.; et al. Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 4, 1190–1209. [Google Scholar] [CrossRef]
- Huang, L.B.; Zhao, L.; Zhang, Y.; Chen, Y.Y.; Zhang, Q.H.; Luo, H.; Zhang, X.; Tang, T.; Gu, L.; Hu, J.S. Self-limited on-site conversion of MoO3 nanodots into vertically aligned ultrasmall monolayer MoS2 for efficient hydrogen evolution. Adv. Energy Mater. 2018, 8, 1800734. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.; Xu, Z.; Wang, X. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal. 2014, 4, 1693–1705. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Liu, W.; Tang, D.; Zhang, B.; Ji, G. A simple hydrothermal process to grow MoS2 nanosheets with excellent dielectric loss and microwave absorption performance. J. Mater. Chem. C 2016, 4, 6816–6821. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of few-layer MoS2 nanosheets: Preparation of luminescent quantum dots via a transition of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.Q.; Lu, M.M.; Li, J.B.; Chen, Z.; Dou, Y.K.; Wang, C.Z.; Rehman, F.; Cao, M.S.; Jin, H.B. Two-dimensional nanosheets of MoS2: A promising material with high dielectric properties and microwave absorption performance. Nanoscale 2015, 7, 15734–15740. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Sun, Y.; Chen, X.; Zheng, J.; Sun, L.; Jiao, L.; Li, J. Metallic and ferromagnetic MoS2 nanobelts with vertically aligned edges. Nano Res. 2015, 9, 2946–2953. [Google Scholar] [CrossRef]
- Lin, H.; Wang, C.; Wu, J.; Xu, Z.; Huang, Y.; Zhang, C. Colloidal synthesis of MoS2 quantum dots: Size-dependent tunable photoluminescence and bioimaging. New J. Chem. 2015, 39, 8492–8497. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Xia, Y. Monodisperse three-layered MoS2 quantum dots as fluorescent reporters for 2,4,6-trinitrotoluene sensing in environmental water and luggage cases. Anal. Chem. 2018, 90, 3942–3949. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J. Facilitated lithium storage in MoS2 overlayers supported on coaxial carbon nanotubes. J. Phys. Chem. C 2007, 4, 1675–1682. [Google Scholar] [CrossRef]
- Wu, F.; Xie, A.; Sun, M.; Wang, Y.; Wang, M. Reduced graphene oxide (RGO) modified spongelike polypyrrole (PPy) aerogel for excellent electromagnetic absorption. J. Mater. Chem. A 2015, 3, 14358–14369. [Google Scholar] [CrossRef]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS2 quantum dots: Ultrasonic preparation, up-conversion and down-conversion bioimaging, and photodynamic therapy. ACS Appl. Mater. Inter. 2016, 8, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Arul, N.S.; Nithya, V.D. Molybdenum disulfide quantum dots: Synthesis and applications. RSC Adv. 2016, 6, 65670–65682. [Google Scholar] [CrossRef]
- Leong, S.X.; Mayorga-Martinez, C.C.; Chia, X.; Luxa, J.; Sofer, Z.; Pumera, M. 2H → 1T phase change in direct synthesis of WS2 nanosheets via solution-based electrochemical exfoliation and their catalytic properties. ACS Appl. Mater. Inter. 2017, 9, 26350–26356. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. J. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Khezri, B.; Eng, A.Y.S.; Sofer, Z.; Ulbrich, P.; Pumera, M. Bipolar electrochemical synthesis of WS2 nanoparticles and their application in magneto-immunosandwich assay. Adv. Funct. Mater. 2016, 26, 4094–4098. [Google Scholar] [CrossRef]
- Liu, H.; Ye, T.; Mao, C. Fluorescent carbon nanoparticles derived from candle soot. Angew. Chem. 2007, 119, 6593–6595. [Google Scholar] [CrossRef]
- Huang, H.; Du, C.; Shi, H.; Feng, X.; Li, J.; Tan, Y.; Song, W. Water-soluble monolayer molybdenum disulfide quantum dots with upconversion fluorescence. Part. Part. Syst. Charact. 2015, 32, 72–79. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, H.; Wu, X.; Pan, W.; Wang, W.; Bai, J.; Zhao, W.; Sun, L.; Wang, X.; Ni, Z. Layer-by-layer thinning of MoS2 by plasma. ACS Nano 2013, 7, 4202. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, Y. Molybdenum disulfide quantum dots as a photoluminescence sensing platform for 2,4,6-trinitrophenol detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, W.H. A standard fluorescence spectrum for calibrating spectro-fluorophotometers. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Vadivelmurugan, A.; Anbazhagan, R.; Tsai, H.C. Preparation of fluorescent MoS2 quantum dots conjugated with various ligands, and its fluorescence imaging. Mater. Lett. 2018, 218, 285–289. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, A.Y.; Huang, D.; Chai, Y.Q.; Zhuo, Y.; Yuan, R. MoS2 quantum dots as new electrochemiluminescence emitters for ultrasensitive bioanalysis of lipopolysaccharide. Anal. Chem. 2017, 89, 8335–8342. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Jia, X.; Xu, B.; Lin, H.; Yang, H.; Song, L.; Wang, X. Amorphous nickel-cobalt complexes hybridized with 1T-phase molybdenum disulfide via hydrazine-induced phase transformation for water splitting. Nat. Commun. 2017, 8, 15377. [Google Scholar] [CrossRef] [PubMed]
- Joensen, P.; Frindt, R.F.; Morrison, S.R. Single-layer MoS2. Mater. Res. Bull. 1986, 21, 457–461. [Google Scholar] [CrossRef]
- Bai, R.; Wang, P.; Fang, Y. Probing microstructures of molybdenum disulfide quantum dots by resonant Raman scattering. Appl. Phys. Lett. 2017, 110, 161910. [Google Scholar] [CrossRef]
- Molina-Sánchez, A.; Wirtz, L. Phonons in single-layer and few-layer MoS2 and WS2. Phys. Rev. B 2011, 84, 155413. [Google Scholar] [CrossRef]
- Ha, H.D.; Han, D.J.; Choi, J.S.; Park, M.; Seo, T.S. Dual role of blue luminescent MoS2 quantum dots in fluorescence resonance energy transfer phenomenon. Small 2014, 10, 3858–3862. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yan, Y.; Cao, X.; Zhang, C.; Ding, C.; Xian, Y. A facile and one-step ethanol-thermal synthesis of MoS2 quantum dots for two-photon fluorescence imaging. J. Mater. Chem. B 2016, 4, 27–31. [Google Scholar] [CrossRef]
- Mouri, S.; Miyauchi, Y.; Matsuda, K. Tunable photoluminescence of monolayer MoS2 via chemical doping. Nano Lett. 2013, 13, 5944–5948. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, G.; Yin, Z.; He, Q.; Li, H.; Zhang, Q.; Zhang, H. Optical identification of single- and few-layer MoS2 sheets. Small 2012, 8, 682–686. [Google Scholar] [CrossRef]
- Clark, R.M.; Carey, B.J.; Daeneke, T.; Atkin, P.; Bhaskaran, M.P.; Latham, K.; Cole, I.S.; Kalantar-zadeh, K. Two-step synthesis of luminescent MoS2–ZnS hybrid quantum dots. Nanoscale 2015, 7, 16763. [Google Scholar] [CrossRef]
- Lin, Y.C.; Dumcenco, D.O.; Huang, Y.S.; Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotechnol. 2014, 9, 391. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Li, C.; Qian, M.; Bu, J.; Wang, J.; Huang, R. Facile growth of well-dispersed and ultra-small MoS2 nanodots in ordered mesoporous silica nanoparticles. Chem. Commun. 2016, 52, 10217–10220. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S. One-step hydrothermal synthesis of monolayer MoS2 quantum dots for highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 10693. [Google Scholar] [CrossRef]
- Xiao, S.J.; Zhao, X.J.; Zuo, J.; Huang, H.Q.; Zhang, L. Highly photoluminescent MoOx quantum dots: Facile synthesis and application in off-on Pi sensing in lake water samples. Anal. Chim. Acta 2016, 906, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Štengl, V.; Henych, J. Strongly luminescent monolayered MoS2 prepared by effective ultrasound exfoliation. Nanoscale 2013, 5, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Ma, H. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew. Chem. 2012, 51, 6432–6435. [Google Scholar] [CrossRef]
- Naito, Y.; Suetake, K. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw. Theory 1971, 19, 65–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Wei, S.; Zhang, B.; Yu, M.; Zhao, W.; Liu, J. Fabrication of porous graphene-Fe3O4 hybrid composites with outstanding microwave absorption performance. Compos. Part A Appl. Sci. 2017, 95, 237–247. [Google Scholar] [CrossRef]
- Xiang, J.; Li, J.; Zhang, X.; Ye, Q.; Xu, J.; Shen, X. Magnetic carbon nanofibers containing uniformly dispersed Fe/Co/Ni nanoparticles as stable and high-performance electromagnetic wave absorbers. J. Mater. Chem. A 2014, 2, 16905–16914. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.S.; Hou, Z.L.; Song, W.L.; Zhang, L.; Lu, M.M.; Jin, H.B.; Fang, X.Y.; Wang, W.Z.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon 2013, 65, 124–139. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Quan, B.; Liang, X.; Shang, C.; Cheng, Y.; Du, Y. Thermal conversion of an Fe3O4@metal–organic framework: A new method for an efficient Fe–Co/nanoporous carbon microwave absorbing material. Nanoscale 2015, 7, 12932–12942. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Liu, M.; Luo, H.; Deng, L.; Huang, L.; Wei, S.; Zhou, C.; Xu, Y. Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring. Nanomaterials 2019, 9, 906. https://doi.org/10.3390/nano9060906
Li Y, Wang X, Liu M, Luo H, Deng L, Huang L, Wei S, Zhou C, Xu Y. Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring. Nanomaterials. 2019; 9(6):906. https://doi.org/10.3390/nano9060906
Chicago/Turabian StyleLi, Yang, Xiaoxia Wang, Mengli Liu, Heng Luo, Lianwen Deng, Lei Huang, Shuang Wei, Congli Zhou, and Yuanhong Xu. 2019. "Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring" Nanomaterials 9, no. 6: 906. https://doi.org/10.3390/nano9060906
APA StyleLi, Y., Wang, X., Liu, M., Luo, H., Deng, L., Huang, L., Wei, S., Zhou, C., & Xu, Y. (2019). Molybdenum Disulfide Quantum Dots Prepared by Bipolar-Electrode Electrochemical Scissoring. Nanomaterials, 9(6), 906. https://doi.org/10.3390/nano9060906