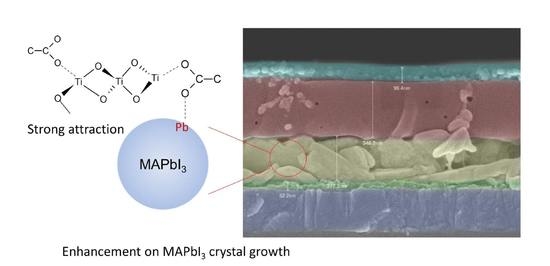
MAPbI3 Incorporated with Carboxyl Group Chelated Titania for Planar Perovskite Solar Cells in Low-Temperature Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. MAPbI3 Solution
2.3. Device Fabrication
2.4. Measurements and Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yu, H.; Liu, X.; Xia, Y.; Dong, Q.; Zhang, K.; Wang, Z.; Zhou, Y.; Song, B.; Li, Y. Room-temperature mixed-solvent-vapor annealing for high performance perovskite solar cells. J. Mater. Chem. A 2016, 4, 321–326. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2014, 9, 106. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.-S.; Chang, J.A.; Lee, Y.H.; Kim, H.-J.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Mukhopadhyay, S.; Gartsman, K.; Hodes, G.; Cahen, D. Elucidating the charge carrier separation and working mechanism of CH3NH3PbI3−xClx perovskite solar cells. Nat. Commun. 2014, 5, 3461. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Jodlowski, A.D.; Roldán-Carmona, C.; Grancini, G.; Salado, M.; Ralaiarisoa, M.; Ahmad, S.; Koch, N.; Camacho, L.; de Miguel, G.; Nazeeruddin, M.K. Large guanidinium cation mixed with methylammonium in lead iodide perovskites for 19% efficient solar cells. Nat. Energy 2017, 2, 972–979. [Google Scholar] [CrossRef] [Green Version]
- De Wolf, S.; Holovsky, J.; Moon, S.-J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.-J.; Yum, J.-H.; Ballif, C. Organometallic halide perovskites: Sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 2014, 5, 1035–1039. [Google Scholar] [CrossRef]
- Yang, H.; Cong, S.; Lou, Y.; Han, L.; Zhao, J.; Sun, Y.; Zou, G. Organic–inorganic hybrid interfacial layer for high-performance planar perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 31746–31751. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shin, I.; Hwang, I.-W.; Kim, S.; Lee, J.; Yang, M.-S.; Jung, Y.K.; Jang, J.-W.; Jeong, J.H.; Park, S.H.; et al. Single-crystal-like perovskite for high-performance solar cells using the effective merged annealing method. ACS Appl. Mater. Interfaces 2017, 9, 12382–12390. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead tri-halide perovskites. Nat. Commun. 2014, 5, 3586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaith, H.J.; Lilliu, S. The Path to Perovskite on Silicon PV. Sci. Video Protoc. 2018, 1, 1. [Google Scholar] [CrossRef]
- Li, N.; Cheng, C.; Wei, H.; Liu, H.; Li, X.; Li, W.; Wang, L. Enhanced efficiency and stability of inverted perovskite solar cells by interfacial engineering with alkyl bisphosphonic molecules. RSC Adv. 2017, 7, 42105–42112. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316. [Google Scholar] [CrossRef]
- Grätzel, M. The rise of highly efficient and stable perovskite solar cells. Acc. Chem. Res. 2017, 50, 487–491. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, B.V. New light on an old story: Perovskites go solar. Angew. Chem. Int. Ed. 2014, 53, 635–637. [Google Scholar]
- Hodes, G. Perovskite-based solar cells. Science 2013, 342, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Azimi, H.; Hou, Y.; Ameri, T.; Przybilla, T.; Spiecker, E.; Kraft, M.; Scherf, U.; Brabec, C.J. Improved high-efficiency perovskite planar heterojunction solar cells via incorporation of a polyelectrolyte interlayer. Chem. Mater. 2014, 26, 5190–5193. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Gowrisanker, S.; Halls, J.J.M.; Laird, D.; Jia, S.; Williams, S.P. Polymer–fullerene bulk-heterojunction solar cells. Adv. Mater. 2010, 22, 3839–3856. [Google Scholar]
- Docampo, P.; Ball, J.M.; Darwich, M.; Eperon, G.E.; Snaith, H.J. Efficient organometal trihalide perovskite planar-heterojunction solar cells on flexible polymer substrates. Nat. Commun. 2013, 4, 2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.nrel.gov/pv/cell-efficiency.html, Best Research-Cell Efficiency Chart (accessed on 22 June 2019).
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar]
- Zhang, W.; Saliba, M.; Moore, D.T.; Pathak, S.K.; Hörantner, M.T.; Stergiopoulos, T.; Stranks, S.D.; Eperon, G.E.; Alexander-Webber, J.A.; Abate, A.; et al. Ultrasmooth organic–Inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells. Nat. Commun. 2015, 6, 6142. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Islam, A.; Yang, X.; Qin, C.; Liu, J.; Zhang, K.; Peng, W.; Han, L. Retarding the crystallization of PbI2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci. 2014, 7, 2934–2938. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.-S.; Wang, H.-H.; Liu, Y.; Li, G.; Yang, Y. Planar heterojunction perovskite solar cells via vapor-assisted solution process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Lee, S.; Kang, R.; Kim, J.-E.; Yeo, J.-S.; Lee, S.-H.; Kim, S.-S.; Yun, J.-M.; Kim, D.-Y. Planar heterojunction perovskite solar cells with superior reproducibility. Sci. Rep. 2014, 4, 6953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-Y.; Chu, C.-Y.; Huang, Y.-C.; Huang, C.-W.; Chang, S.-Y.; Chen, C.-A.; Chao, C.-Y.; Su, W.-F. Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interfaces 2015, 7, 4955–4961. [Google Scholar] [CrossRef] [PubMed]
- Manda, X.; Fuzhi, H.; Wenchao, H.; Yasmina, D.; Ye, Z.; Joanne, E.; Angus, G.W.; Udo, B.; Yi-Bing, C.; Leone, S. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, M.; Wu, W.; Vasiliev, A.L.; Zhu, K.; Padture, N.P. Room-temperature crystallization of hybrid-perovskite thin films via solvent-solvent extraction for high-performance solar cells. J. Mater. Chem. A 2015, 3, 8178–8184. [Google Scholar] [CrossRef]
- Liu, B.-T.; Chou, Y.-H.; Liu, J.-Y. Extremely enhanced photovoltaic properties of dye-sensitized solar cells by sintering mesoporous TiO2 photoanodes with crystalline titania chelated by acetic acid. J. Power Sources 2016, 310, 79–84. [Google Scholar] [CrossRef]
- Liu, B.-T.; Liou, J.-Y. High efficiency of dye-sensitized solar cells with two-layer mesoporous photoanodes fabricated in a low temperature process. Electrochim. Acta 2018, 261, 421–427. [Google Scholar] [CrossRef]
- Liu, B.T.; Tang, S.J.; Yu, Y.Y.; Lin, S.H. High-refractive-index polymer/inorganic hybrid films containing high TiO2 contents. Colloids Surf. A 2011, 377, 138–143. [Google Scholar] [CrossRef]
- Haase, M.; Weller, H.; Henglein, A. Photochemistry and radiation chemistry of colloidal semiconductors. 23. Electron storage on zinc oxide particles and size quantization. J. Phys. Chem. 1988, 92, 482–487. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Kemerink, M.; Yang, X.; Janssen, R.A. Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B 2005, 109, 9505–9516. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Tang, Z.; Zhao, Y.; Zhong, X.; Venkatesan, S.; Graham, H.; Patton, M.; Jing, Y.; Guloy, A.M.; Yao, Y. Solvent engineering towards controlled grain growth in perovskite planar heterojunction solar cells. Nanoscale 2015, 7, 10595–10599. [Google Scholar] [CrossRef] [PubMed]
- Shirayama, M.; Kato, M.; Miyadera, T.; Sugita, T.; Fujiseki, T.; Hara, S.; Kadowaki, H.; Murata, D.; Chikamatsu, M.; Fujiwara, H. Degradation mechanism of CH3NH3PbI3 perovskite materials upon exposure to humid air. J. Appl. Phys. 2016, 119, 115501. [Google Scholar] [CrossRef]
- Huang, X.; Hu, Z.; Xu, J.; Wang, P.; Zhang, J.; Zhu, Y. Low-temperature processed ultrathin TiO2 for efficient planar heterojunction perovskite solar cells. Electrochim. Acta 2017, 231, 77–84. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Cai, Y.; Chen, J.; Wang, J.; Huang, R.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; et al. Enhanced performance of CH3NH3PbI3−xClx perovskite solar cells by CH3NH3I modification of TiO2-perovskite layer interface. Nanoscale Res. Lett. 2016, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, Y.; Zhao, Y.L.; Che, M.; Zhu, L.; Gu, X.Q.; Qiang, Y.H. Morphology modification of perovskite film by a simple post-treatment process in perovskite solar cell. Mater. Sci. Eng. B 2017, 217, 18–25. [Google Scholar] [CrossRef]
- Guohua, D.; Tengling, Y.; Yulin, Y.; Li, S.; Debin, X.; Junhai, W.; Xiao, F.; Ruiqing, F. SiW12–TiO2 mesoporous layer for enhanced electron-extraction efficiency and conductivity in perovskite solar cells. ChemSusChem 2017, 10, 2218–2225. [Google Scholar]
- Jeong, I.; Park, Y.H.; Bae, S.; Park, M.; Jeong, H.; Lee, P.; Ko, M.J. Solution-processed ultrathin TiO2 compact layer hybridized with mesoporous TiO2 for high-performance perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 36865–36874. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-X.; Ge, Q.-Q.; Xue, D.-J.; Ding, J.; Ma, J.-Y.; Chen, Y.-X.; Zhang, B.; Feng, Y.; Wan, L.-J.; Hu, J.-S. Tuning the Fermi-level of TiO2 mesoporous layer by lanthanum doping towards efficient perovskite solar cells. Nanoscale 2016, 8, 16881–16885. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Jalebi, M.; Dar, M.I.; Sadhanala, A.; Senanayak, S.P.; Giordano, F.; Zakeeruddin, S.M.; Grätzel, M.; Friend, R.H. Impact of a mesoporous titania–perovskite interface on the performance of hybrid organic–inorganic perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.F.; Kwon, H.-C.; Yang, W.; Mane, R.S.; Moon, J. Performance enhancement of mesoporous TiO2-based perovskite solar cells by ZnS ultrathin-interfacial modification layer. J. Alloys Compd. 2018, 738, 405–414. [Google Scholar] [CrossRef]
- Giordano, F.; Abate, A.; Correa Baena, J.P.; Saliba, M.; Matsui, T.; Im, S.H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Hagfeldt, A.; Graetzel, M. Enhanced electronic properties in mesoporous TiO2 via lithium doping for high-efficiency perovskite solar cells. Nat. Commun. 2016, 7, 10379. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, J.; Xiao, L.; Chen, J.; Zhang, B.; Yao, J.; Dai, S. Influence of the porosity of the TiO2 film on the performance of the perovskite solar cell. Int. J. Photoenergy 2017, 2017, 10. [Google Scholar] [CrossRef]
- Dagar, J.; Castro-Hermosa, S.; Gasbarri, M.; Palma, A.L.; Cina, L.; Matteocci, F.; Calabrò, E.; Di Carlo, A.; Brown, T.M. Efficient fully laser-patterned flexible perovskite modules and solar cells based on low-temperature solution-processed SnO2/mesoporous-TiO2 electron transport layers. Nano Res. 2018, 11, 2669–2681. [Google Scholar] [CrossRef]
- Ravishankar, S.; Gharibzadeh, S.; Roldán-Carmona, C.; Grancini, G.; Lee, Y.; Ralaiarisoa, M.; Asiri, A.M.; Koch, N.; Bisquert, J.; Nazeeruddin, M.K. Influence of charge transport layers on open-circuit voltage and hysteresis in perovskite solar cells. Joule 2018, 2, 788–798. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Layani, M.; Magdassi, S.; Etgar, L. A mesoporous-planar hybrid architecture of methylammonium lead iodide perovskite based solar cells. J. Mater. Chem. A 2016, 4, 14423–14429. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Xu, S.-B.; Deng, J.-G.; Gao, L.-Z. Enhancing the efficiency of planar heterojunction perovskite solar cells via interfacial engineering with 3-aminopropyl trimethoxy silane hydrolysate. Roy. Soc. Open Sci. 2017, 4, 170980. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Zhu, L.; Luo, Y.; Li, D.; Wu, H.; Meng, Q. Polystyrene stabilized perovskite component, grain and microstructure for improved efficiency and stability of planar solar cells. Nano Energy 2018, 43, 383–392. [Google Scholar] [CrossRef]







| Samples | h-TAc Content, wt% | Average Crystal Size, nm | Voc, V | Jsc, mA cm−2 | FF, % | η, % | R1, Ω | R2, Ω | R3, Ω |
|---|---|---|---|---|---|---|---|---|---|
| PVSC | 0 | 223.2 ± 42.9 | 1.02 | 16.0 | 57.8 | 9.5 | 50.0 | 301.6 | 645.6 |
| PVSC-hTAc75 | 0.75 | 264.9 ± 58.1 | 0.99 | 16.9 | 60.0 | 10.0 | 41.0 | 355.1 | 2689.0 |
| PVSC-hTAc85 | 0.85 | 293.5 ± 34.6 | 1.02 | 22.69 | 68.6 | 15.9 | 17.0 | 379.1 | 6143.0 |
| PVSC-hTAc100 | 1.00 | 243.8 ± 53.4 | 0.99 | 19.5 | 52.6 | 10.0 | 1.7 | 483.7 | 2284.0 |
| Samples | the Mesoporous Layer | Voc, V | Jsc, mA cm−2 | FF, % | η, % | R1, Ω | R2, Ω | R3, Ω |
|---|---|---|---|---|---|---|---|---|
| PVSC-meso | h-TAc | 1.00 | 13.5 | 53.3 | 7.2 | 37.0 | 127.7 | 1793.0 |
| Samples | Kinds of TiO2 | Average Crystal Size, nm | Voc, V | Jsc, mA cm−2 | FF, % | η, % | R1, Ω | R2, Ω | R3, Ω |
|---|---|---|---|---|---|---|---|---|---|
| PVSC-P25 | P25 | 254.2 ± 66.3 | 0.97 | 15.5 | 68.8 | 10.4 | 44.9 | 468.0 | 2741.0 |
| PVSC-ST01 | ST-01 | 213.9 ± 40.0 | 0.97 | 15.4 | 67.2 | 10.1 | 22.0 | 517.0 | 2566.0 |
| PVSC-18NRT | 18NR-T | 215.6 ± 37.5 | 0.86 | 10.3 | 56.5 | 5.0 | 2.2 | 404.6 | 590.1 |
| TiO2 Samples | h-TAc | P25 | ST-01 | 18NR-T |
|---|---|---|---|---|
| Lead ion, ppm | 51.22 | 7.17 | 6.96 | 7.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.-S.; Balamurugan, R.; Liu, B.-T.; Lee, R.-H.; Chou, H.-T. MAPbI3 Incorporated with Carboxyl Group Chelated Titania for Planar Perovskite Solar Cells in Low-Temperature Process. Nanomaterials 2019, 9, 908. https://doi.org/10.3390/nano9060908
Li P-S, Balamurugan R, Liu B-T, Lee R-H, Chou H-T. MAPbI3 Incorporated with Carboxyl Group Chelated Titania for Planar Perovskite Solar Cells in Low-Temperature Process. Nanomaterials. 2019; 9(6):908. https://doi.org/10.3390/nano9060908
Chicago/Turabian StyleLi, Pei-Shan, Rathinam Balamurugan, Bo-Tau Liu, Rong-Ho Lee, and Hsueh-Tao Chou. 2019. "MAPbI3 Incorporated with Carboxyl Group Chelated Titania for Planar Perovskite Solar Cells in Low-Temperature Process" Nanomaterials 9, no. 6: 908. https://doi.org/10.3390/nano9060908