Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SnO2 Nanoneedle-anchored NiO Microsphere
2.2. Characterization
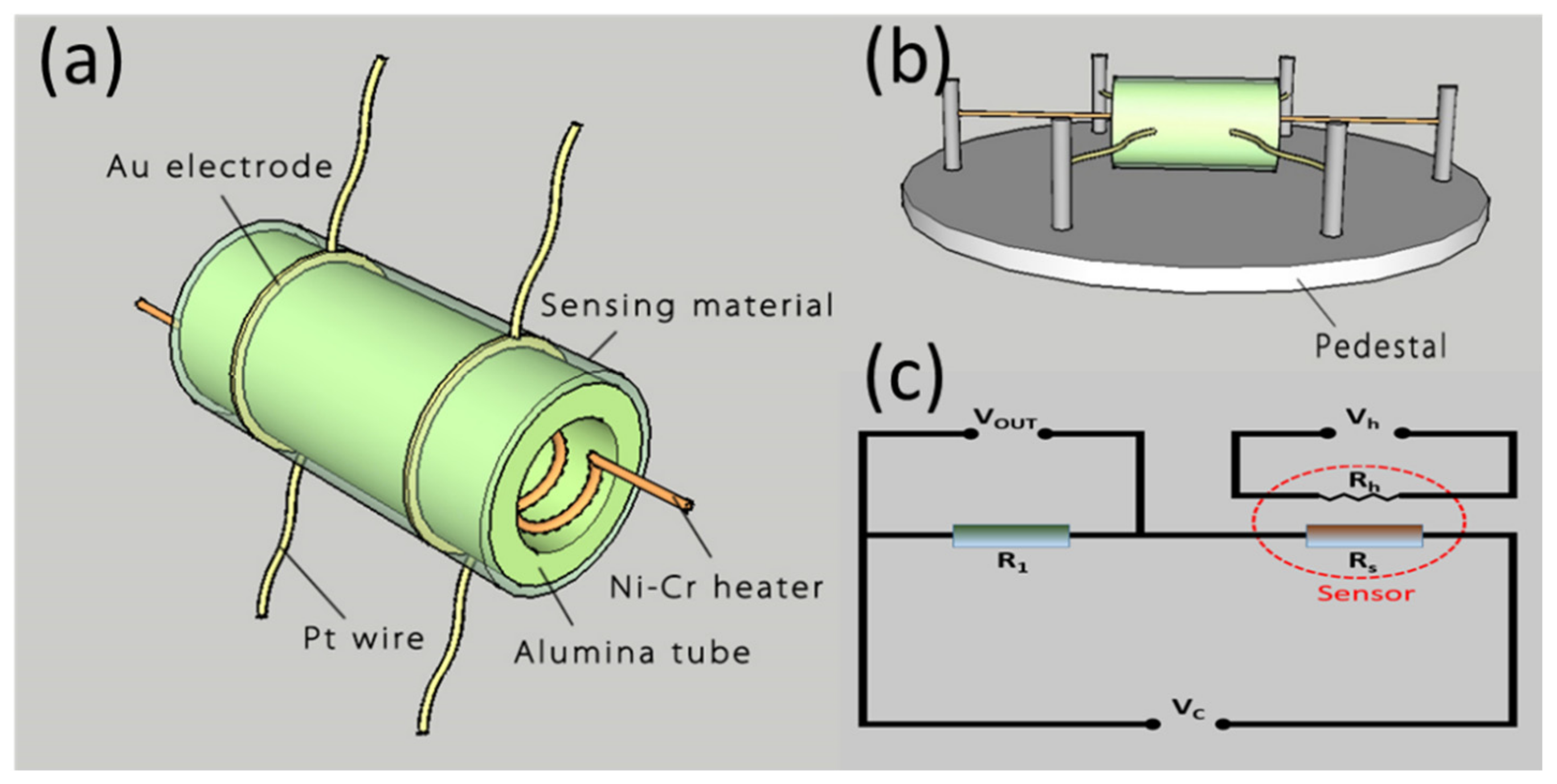
2.3. Fabrication and Measurement of Gas Sensors
3. Results and Discussion
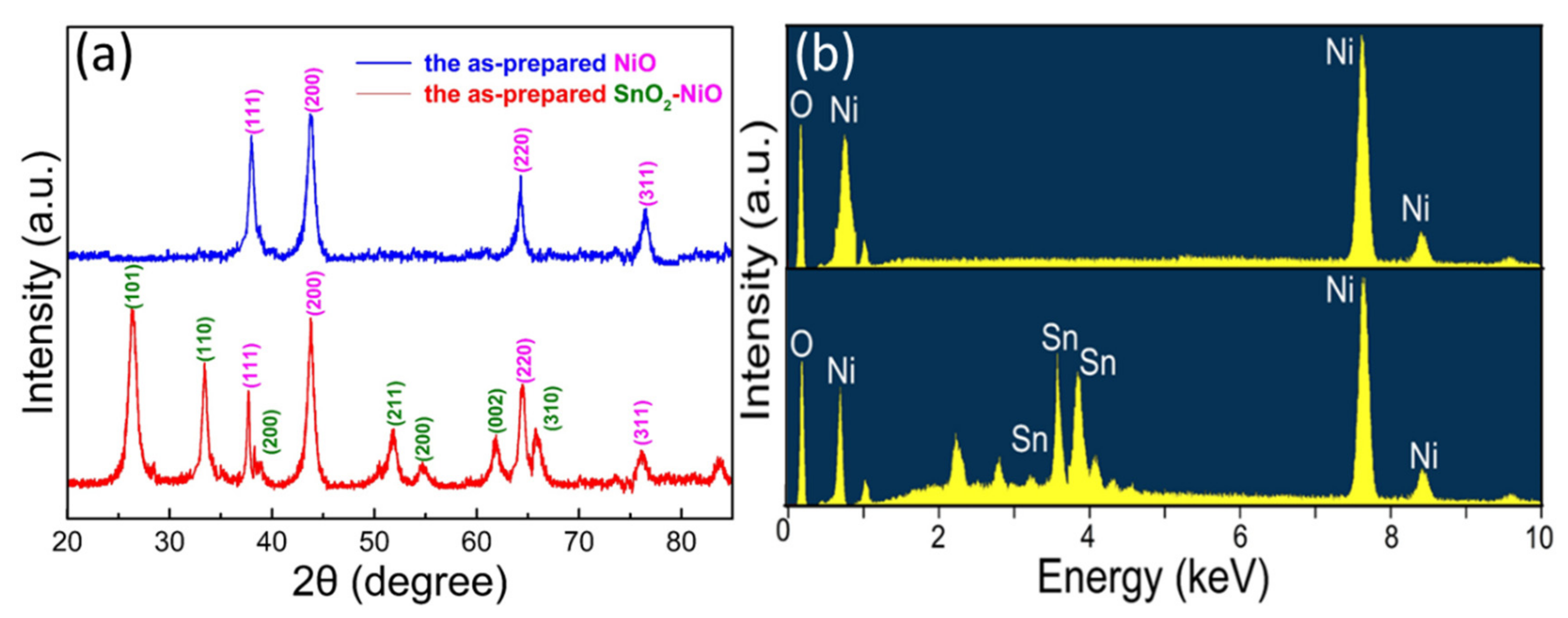
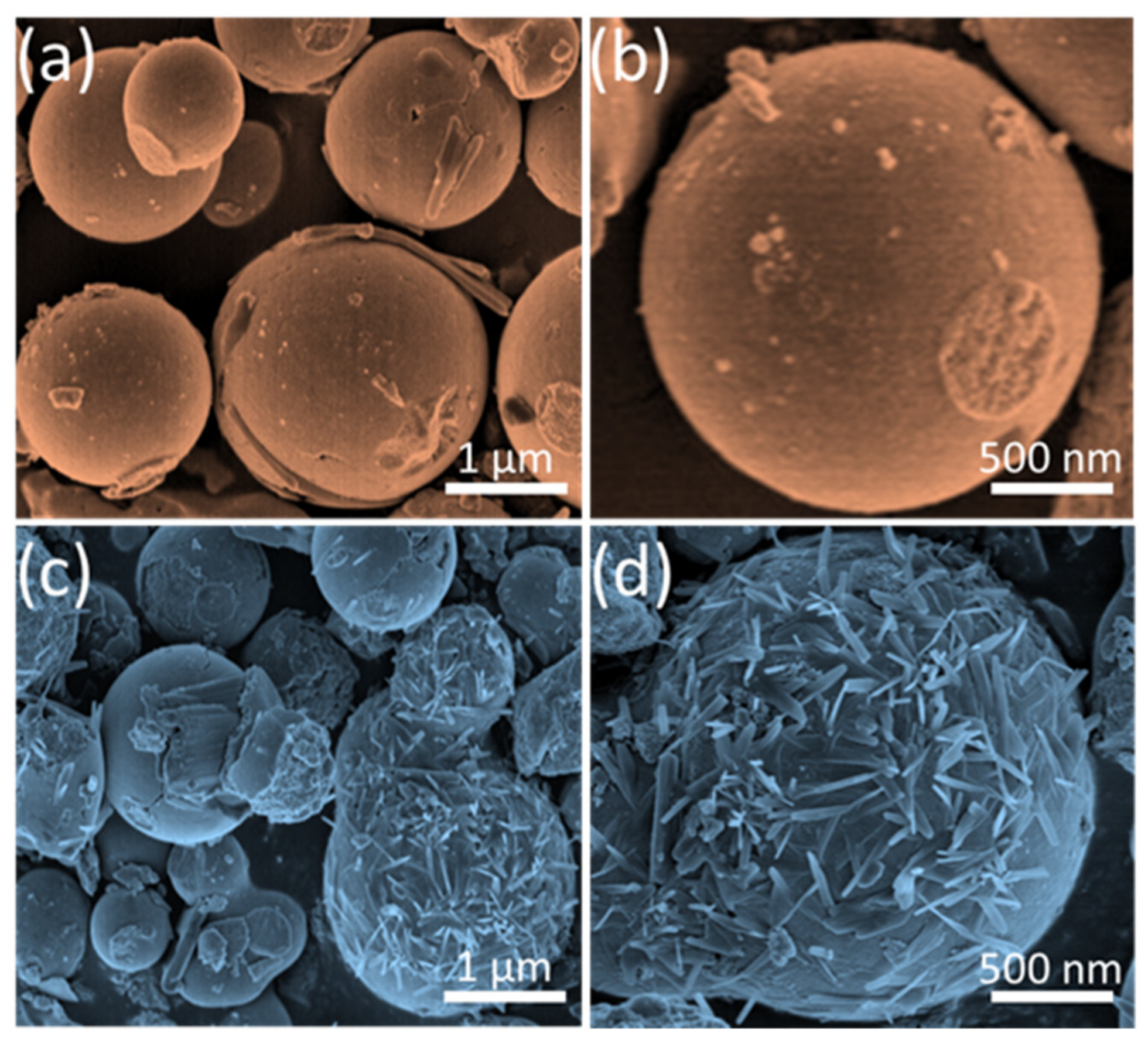
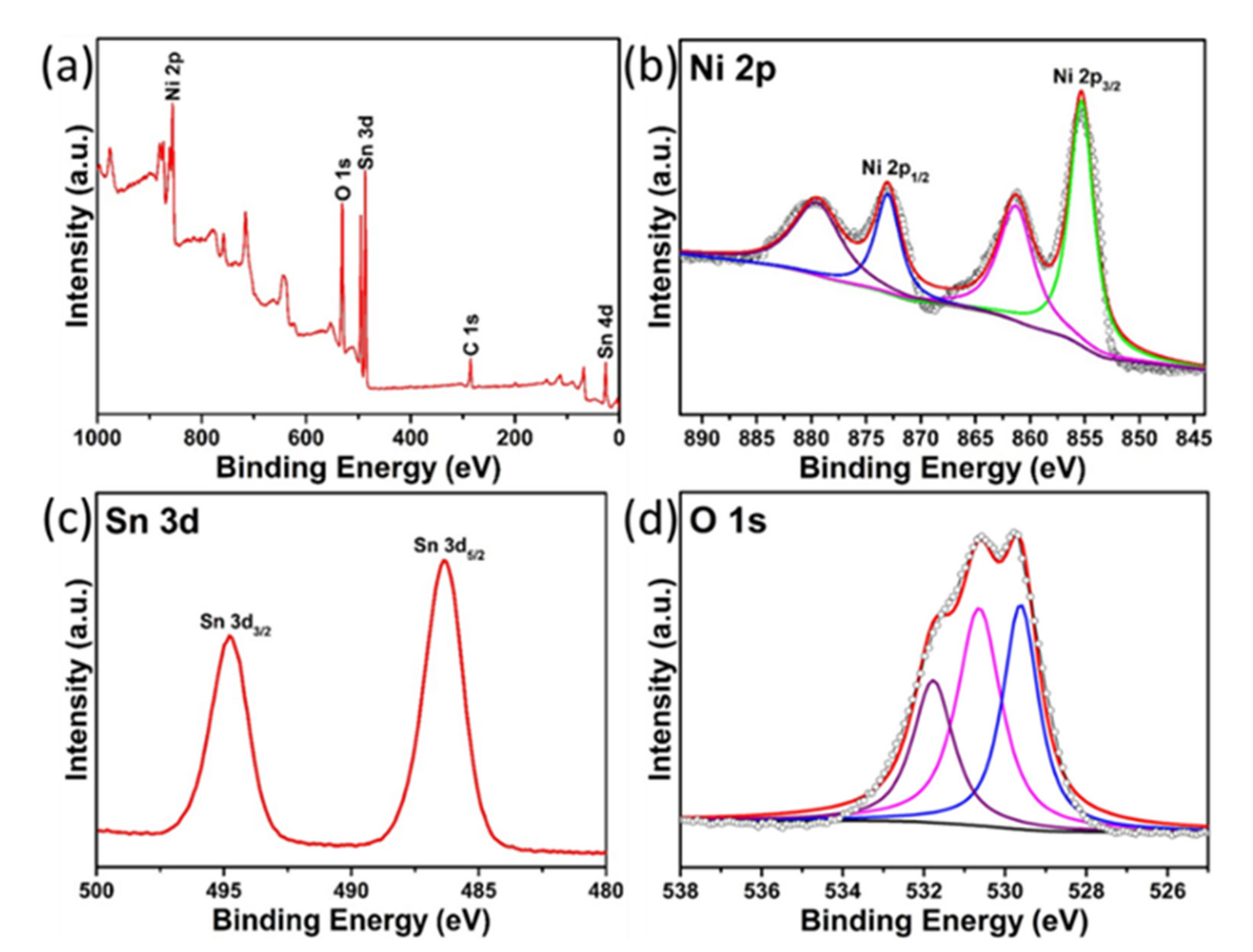
3.1. Structural and Morphological Analysis
3.2. Growth Mechanism
3.3. Gas Sensing Properties
3.4. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.X.; Dong, X.C.; Gui, Y.G. Theoretical and experimental study on competitive adsorption of SF6 decomposed components on Au-modified anatase (101) surface. Appl. Surf. Sci. 2016, 387, 437–445. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Jiang, C.X.; Li, P.; Sun, Y.E. Layer-by-layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet based nanocomposite toward high performance ammonia detection. ACS Appl. Mater. Inter. 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B-Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Zhang, X.X.; Gui, Y.G.; Dong, X.C. Preparation and application of TiO2 nanotube array gas sensor for SF6-insulated equipment detection: A review. Nanoscale Res. Lett. 2016, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Z.; Wu, Z.L.; Zong, X.Q. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B-Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Zhou, H.; Zou, C.W.; Xu, K.; Luo, X.F.; Yu, T.; Zhang, W.; Liu, Y.T.; Yuan, C.L. A systematic study on the crystal facets-dependent gas sensing properties of anatase TiO2 with designed {010}, {101} and {001} facets. Ceram. Int. 2019, 45, 6282–6290. [Google Scholar] [CrossRef]
- Chinh, N.D.; Kim, C.; Kim, D. UV-light-activated H2S gas sensing by a TiO2 nanoparticulate thin film at room temperature. J. Alloy. Compd. 2019, 778, 247–255. [Google Scholar] [CrossRef]
- Zhao, G.D.; Xuan, J.Y.; Liu, X.L.; Jia, F.C.; Sun, Y.P.; Sun, M.L.; Yin, G.C.; Liu, B. Low-Cost and High-Performance ZnO Nanoclusters Gas Sensor Based on New-Type FTO Electrode for the Low-Concentration H2S Gas Detection. Nanomaterials 2019, 9, 435. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Peng, S.D. Hydrothermal Synthesis of Various Hierarchical ZnO Nanostructures and Their Methane Sensing Properties. Sensors 2013, 13, 6171–6182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Zeng, W.; Li, Y.Q. NO2 and H2 sensing properties for urchin-like hexagonal WO3 based on experimental and first-principle investigations. Ceram. Int. 2019, 45, 6043–6050. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Zeng, W.; Li, Y.Q. A novel seawave-like hierarchical WO3 nanocomposite and its ammonia gas properties. Mater. Lett. 2019, 248, 86–88. [Google Scholar] [CrossRef]
- Zhou, Q.; Umar, A.; Sodki, E.; Amine, A.; Xu, L.N.; Gui, Y.G.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Fabrication and characterization of highly sensitive and selective sensors based on porous NiO nanodisks. Sens. Actuators B-Chem. 2018, 259, 604–615. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, Q.; Wang, J.X.; Gui, Y.G.; Zeng, W. A novel porous NiO nanosheet and its H2 sensing performance. Mater. Lett. 2019, 245, 166–169. [Google Scholar] [CrossRef]
- Guan, X.F.; Wang, Y.J.; Luo, P.H.; Yu, Y.L.; Chen, D.G.; Li, X.Y. Incorporating N Atoms into SnO2 Nanostructure as an Approach to Enhance Gas Sensing Property for Acetone. Nanomaterials 2019, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Kumar, R.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Zhu, S.P. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Yu, K.L.; Hu, J.X.; Li, X.H.; Zhang, L.C.; Lv, Y. Camellia-like NiO: A novel cataluminescence sensing material for H2S. Sens. Actuators B-Chem. 2019, 288, 243–250. [Google Scholar] [CrossRef]
- Zhang, S.S.; Li, Y.W.; Sun, G.; Zhang, B.; Wang, Y.; Cao, J.L.; Zhang, Z.Y. Enhanced methane sensing properties of porous NiO nanaosheets by decorating with SnO2. Sens. Actuators B-Chem. 2019, 288, 373–382. [Google Scholar] [CrossRef]
- Kuang, C.W.; Zeng, W.; Ye, H.; Li, Y.Q. A novel approach for fabricating NiO hollow spheres for gas sensors. Physica E 2018, 97, 314–316. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, Q.; Lu, Z.R.; Xu, L.N.; Gui, Y.G.; Tang, C. Morphology controllable synthesis of hierarchical WO3 nanostructures and C2H2 sensing properties. Physica E 2019, 109, 253–260. [Google Scholar] [CrossRef]
- Xu, L.N.; Zeng, W.; Li, Y.Q. Synthesis of morphology and size-controllable SnO2 hierarchical structures and their gas-sensing performance. Appl. Surf. Sci. 2018, 457, 1064–1071. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhang, D.Z.; Yin, N.L.; Yao, Y.; Shaymurat, T.; Zhou, X.Y. Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film. Nanomaterials 2017, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Y.Q.; Zeng, W. Enhanced ethanol sensing and mechanism of Cr-doped ZnO nanorods: Experimental and computational study. Ceram. Int. 2017, 43, 14873–14879. [Google Scholar] [CrossRef]
- Xing, X.X.; Yang, Y.; Yan, Z.Y.; Hu, Y.; Zou, T.; Wang, Z.D.; Wang, Y.D. CdO-Ag-ZnO nanocomposites with hierarchically porous structure for effective VOCs gas-sensing properties. Ceram. Int. 2019, 45, 4322–4334. [Google Scholar] [CrossRef]
- Drmosh, Q.A.; Hendi, A.H.; Hossain, M.K.; Yamani, Z.H.; Moqbel, R.A.; Hezam, A.; Gondal, M. UV-activated gold decorated rGO/ZnO heterostructured nanocomposite sensor for efficient room temperature H2 detection. Sens. Actuators B-Chem. 2019, 290, 666–675. [Google Scholar] [CrossRef]
- Karouei, S.F.H.; Moghaddam, H.M. P-p heterojunction of polymer/hierarchical mesoporous LaFeO3 microsphere as CO2 gas sensing under high humidity. Appl. Surf. Sci. 2019, 479, 1029–1038. [Google Scholar] [CrossRef]
- Sun, G.J.; Kheel, H.; Park, S.; Lee, S.; Park, S.E.; Lee, C. Synthesis of TiO2 nanorods decorated with NiO nanopartieles and their acetone sensing properties. Ceram. Int. 2016, 42, 1063–1069. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W.; Yang, J.D.; Li, Y.Q. One-step hydrothermal fabrication of nanosheet-assembled NiO/ZnO microflower and its ethanol sensing property. Ceram. Int. 2018, 44, 19825–19830. [Google Scholar] [CrossRef]
- Bunpang, K.; Wisitsoraat, A.; Tuantranont, A.; Singkammo, S.; Phanichphant, S.; Liewhiran, C. Highly selective and sensitive CH4 gas sensors based on flame-spray-made Cr-doped SnO2 particulate films. Sens. Actuators B-Chem. 2019, 291, 177–191. [Google Scholar] [CrossRef]
- Lu, Z.R.; Zhou, Q.; Xu, L.N.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Chen, W.G. Synthesis and Characterization of Highly Sensitive Hydrogen (H2) Sensing Device Based on Ag Doped SnO2 Nanospheres. Materials 2018, 11, 492. [Google Scholar] [CrossRef]
- Bai, S.; Fu, H.; Zhao, Y.; Tian, K.; Luo, R.; Li, D.; Chen, A. On the construction of hollow nanofibers of ZnO-SnO2 heterojunctions to enhance the NO2 sensing properties. Sens. Actuators B-Chem. 2018, 266, 692–702. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Wang, W.D.; Xue, Y.; Sun, Y.J.; Li, P.W.; Lian, K.; Zhang, W.D.; Chen, L.; Shi, J.; et al. Synthesis and gas sensing properties of NiO/SnO2 hierarchical structures toward ppb-level acetone detection. Mater. Res. Bull. 2018, 102, 294–303. [Google Scholar] [CrossRef]
- Bai, S.L.; Liu, C.Y.; Luo, R.X.; Chen, A.F. Metal organic frameworks-derived sensing material of SnO2/NiO composites for detection of triethylamine. Appl. Surf. Sci. 2018, 437, 304–313. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B-Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.Y.; Wang, G.S.; Shen, Y.B.; San, X.G.; Li, M.; Meng, F.L. Low-temperature formaldehyde gas sensors based on NiO-SnO2 heterojunction microflowers assembled by thin porous nanosheets. Sens. Actuators B-Chem. 2018, 273, 418–428. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W. Shape control of Co3O4 micro-structures for high-performance gas sensor. Physica E 2018, 95, 121–124. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zeng, W.; Li, Y.Q. Theoretical and experimental investigations on H2 sensing properties of flower-like titanium dioxide. Mater. Res. Bull. 2018, 107, 139–146. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhou, Q.; Zhang, Q.Y.; Hong, C.X.; Xu, L.N.; Jin, L.F.; Chen, W.G. Synthesis, Characterization and Enhanced Sensing Properties of a NiO/ZnO p-n Junctions Sensor for the SF6 Decomposition Byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 913. [Google Scholar] [CrossRef] [PubMed]
- Varshney, D.; Dwivedi, S. Synthesis, structural, Raman spectroscopic and paramagnetic properties of Sn doped NiO nanoparticles. Superlattice. Microst. 2015, 86, 430–437. [Google Scholar] [CrossRef]
- He, J.; Jiao, W.L.; Zhang, L.; Feng, R. Preparation and gas-sensing performance of SnO2/NiO composite oxide synthesized by microwave-assisted liquid phase deposition. Particuology 2018, 41, 118–125. [Google Scholar] [CrossRef]
- Bai, S.L.; Liu, J.C.; Guo, J.; Luo, R.X.; Li, D.Q.; Song, Y.J.; Liu, C.C.; Chen, A.F. Facile preparation of SnO2/NiO composites and enhancement of sensing performance to NO2. Sens. Actuators B-Chem. 2017, 249, 22–29. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525–8531. [Google Scholar] [CrossRef]
- Mane, A.T.; Navale, S.T.; Sen, S.; Aswal, D.K.; Cupta, S.K.; Patil, V.B. Nitrogen dioxide (NO2) sensing performance of p-polypyrrole/n-tungsten oxide hybrid nanocomposites at room temperature. Org. Electron. 2015, 16, 195–204. [Google Scholar]
- Karmakar, N.; Fernandes, R.; Jain, S.; Patil, U.V.; Shimpi, N.G.; Bhat, N.V.; Kothari, D.C. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sens. Actuators B-Chem. 2017, 242, 118–126. [Google Scholar] [CrossRef]
- Jain, S.; Karmakar, N.; Shah, A.; Shimpi, N.G. Development of Ni doped ZnO/polyaniline nanocomposites as high response room temperature NO2 sensor. Mat. Sci. Eng. B 2019, 247, 114381. [Google Scholar] [CrossRef]
- Haiduk, Y.S.; Khort, A.A.; Lapchuk, N.M.; Savitsky, A.A. Study of WO3–In2O3 nanocomposites for highly sensitive CO and NO2 gas sensors. J. Solid. State. Chem. 2019, 273, 25–31. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, W.; Li, Y.Q. Hydrothermal synthesis and controlled growth of hierarchical 3D flowerlike MoS2 nanospheres assisted with CTAB and their NO2 gas sensing. Appl. Surf. Sci. 2018, 455, 276–282. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, L.X.; Bai, S.L.; Fu, H.; Guo, J.; Feng, Y.J.; Luo, R.X.; Li, D.Q.; Chen, A.F. Pyrolyzing Co/Zn bimetallic organic framework to form p-n heterojunction of Co3O4/ZnO for detection of formaldehyde. Sens. Actuators B-Chem. 2019, 285, 291–301. [Google Scholar] [CrossRef]





| Materials | Concentration (ppm) | Operating Temperature (oC) | Response | Ref. |
|---|---|---|---|---|
| WO3 nanorods/graphene nanocomposites | 1 | 300 | 5 | [41] |
| Ppy–WO3 nanocomposites | 100 | R.T. | 1.61 | [42] |
| PTSA doped Ag-PPy nanocomposites | 100 | R.T. | 1.68 | [43] |
| Ni@ZnO/PANi nanocomposites | 100 | R.T. | 1.75 | [44] |
| WO3–In2O3 nanocomposites | 1 | 140 | 2 | [45] |
| SnO2/NiO nanocomposites | 20 | 230 | 14.45 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Zhou, Q.; Wang, J.; Lu, Z.; Xu, L.; Zeng, W. Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances. Nanomaterials 2019, 9, 1015. https://doi.org/10.3390/nano9071015
Wei Z, Zhou Q, Wang J, Lu Z, Xu L, Zeng W. Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances. Nanomaterials. 2019; 9(7):1015. https://doi.org/10.3390/nano9071015
Chicago/Turabian StyleWei, Zhijie, Qu Zhou, Jingxuan Wang, Zhaorui Lu, Lingna Xu, and Wen Zeng. 2019. "Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances" Nanomaterials 9, no. 7: 1015. https://doi.org/10.3390/nano9071015
APA StyleWei, Z., Zhou, Q., Wang, J., Lu, Z., Xu, L., & Zeng, W. (2019). Hydrothermal Synthesis of SnO2 Nanoneedle-Anchored NiO Microsphere and its Gas Sensing Performances. Nanomaterials, 9(7), 1015. https://doi.org/10.3390/nano9071015







