Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro
Abstract
1. Introduction
2. Results
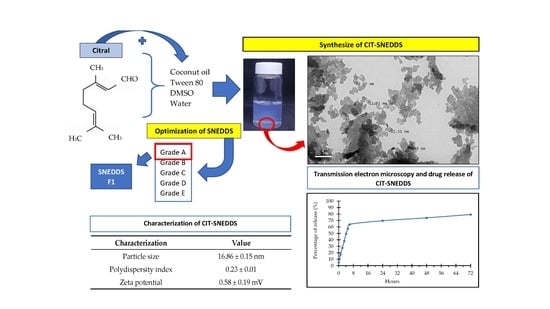
2.1. Preparation of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
2.1.1. Process Parameter Optimization of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
2.1.2. Stability Assessment of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
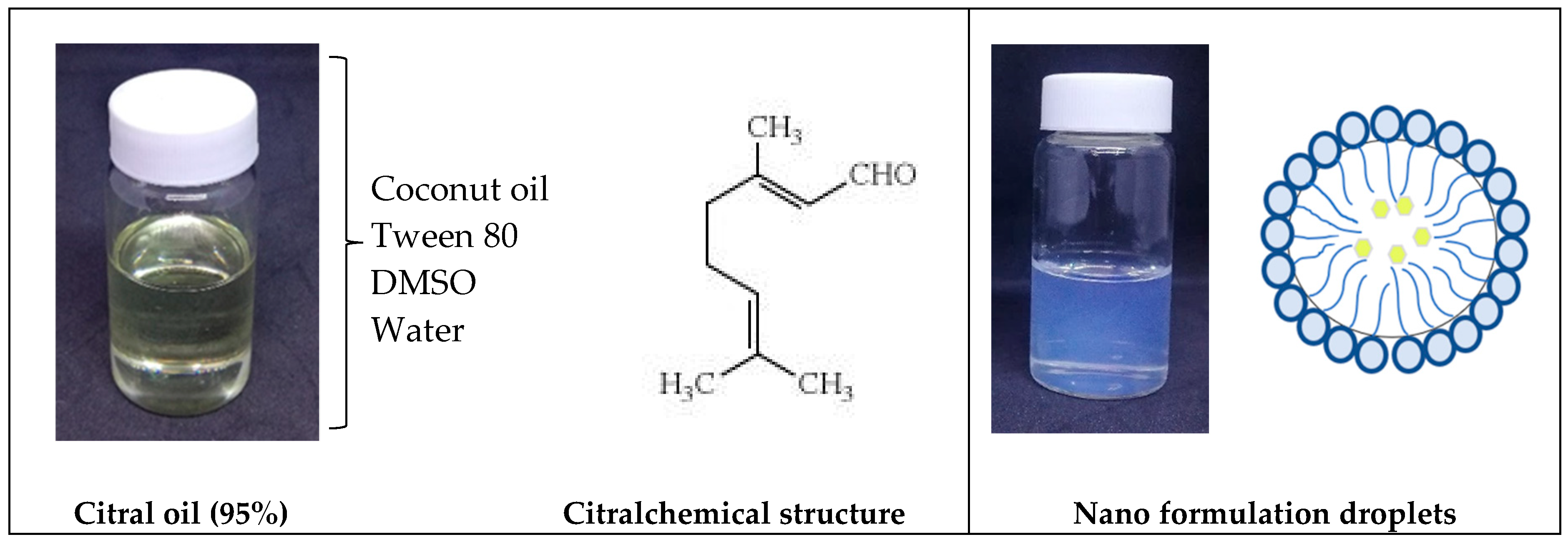
2.2. Synthesis and Formulation of Citral Loaded Nano-Emulsifying Drug Delivery System (CIT-SNEDDS)
2.3. Physicochemical Characterization of Citral Loaded Self Nano-Emulsifying Drug Delivery System (CIT-SNEDDS) Formulation
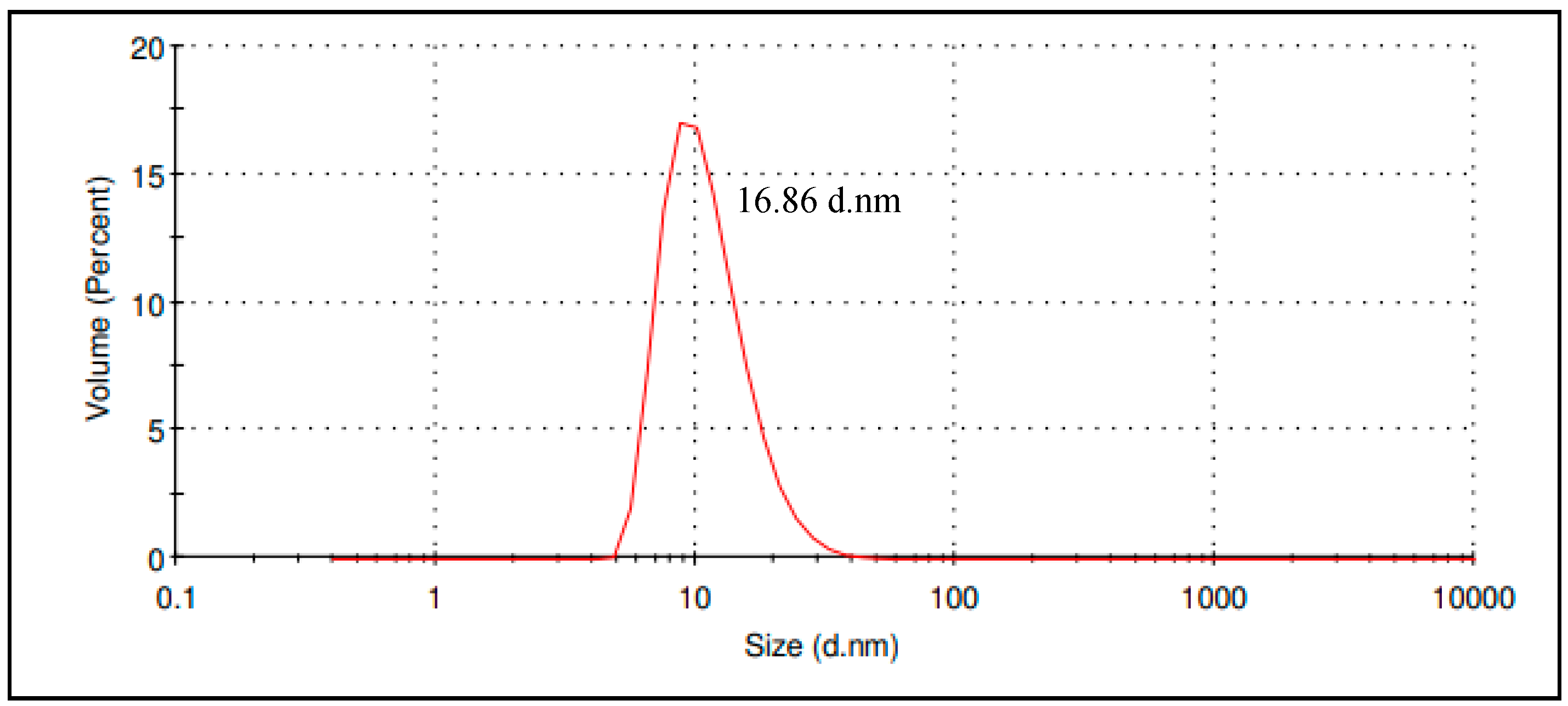
2.3.1. Particle Size and Polydispersity Index
2.3.2. CIT-SNEDDS Surface Charge Analysis
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. In Vitro Drug Releasing Study
2.4. In Vitro Cytotoxicity of CIT-SNEDDS Formulation
Antiproliferative Effect of CIT-SNEDDS on HT29 and SW620 Colon Cancer Cells
3. Discussion
3.1. Preparation of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
3.1.1. Process Parameter Optimization of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
3.1.2. Stability Assessment of Self Nano-Emulsifying Drug Delivery System (SNEDDS)
3.2. Synthesize and Formulation of Citral Loaded Self Nano-Emulsifying Drug Delivery System (CIT-SNEDDS)
3.3. Physicochemical Characterization of Citral Loaded Self Nano-Emulsifying Drug Delivery System (CIT-SNEDDS) Formulation
3.3.1. Particle Size and Polydispersity Index
3.3.2. CIT-SNEDDS Surface Charge Analysis
3.3.3. Transmission Electron Microscopy (TEM)
3.3.4. In Vitro Drug Releasing Study
3.4. In Vitro Cytotoxicity of CIT-SNEDD Formulation
Antiproliferative Effect of CIT-SNEDD on HT29 and SW620 Colon Cancer Cells
4. Materials and Methods
4.1. Materials
4.2. Process Parameter Optimization of SNEDDS
- Grade A: Rapidly forming emulsion, with a clear or bluish appearance
- Grade B: Rapidly forming with slightly less clear emulsion, with a bluish white appearance
- Grade C: Fine milky emulsion
- Grade D: Slow to emulsify, dull, greyish white emulsion having slightly oily appearance
- Grade E: Poor or minimal emulsification with large oil droplets on the surface
4.3. Synthesize and Formulation of CIT-SNEDDS
4.4. Characterization of CIT-SNEDDS
4.4.1. Particle Size and Polydispersity Index
4.4.2. CIT-SNEDDS Surface Charge Analysis
4.4.3. Transmission Electron Microscopy
4.4.4. In Vitro Drug Release Study
4.5. In Vitro Cytotoxicity Study
4.5.1. Cell Culture
4.5.2. Cytotoxic Assay (MTT) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dudai, N.; Weinstein, Y.; Krup, M.; Rabinski, T.; Ofir, R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005, 71, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; Felder, M.; Fass, L.; Kaur, J.; Czarnecki, A.; Rathi, K.; Zeng, S.; Osowski, K.K.; Howell, C.; Xiong, M.P.; et al. Modulation of oxidative stress and subsequent induction of apoptosis and endoplasmic reticulum stress allows citral to decrease cancer cell proliferation. Sci. Rep. 2016, 6, 27530. [Google Scholar] [CrossRef]
- Martins, H.B.; Selis, N.N.; Souze, C.L.S.; Nascimento, F.S.; Carvalho, S.P.; Gusmao, L.D.; Nascimento, J.S.; Brito, A.K.P.; Souza, S.I.; Olveira, M.V.; et al. Anti-Inflammatory Activity of the Essential Oil Citral in Experimental Infection with Staphylococcus aureus in a Model Air Pouch. Evid. -Based Complement. Altern. Med. 2017, 2017, 2505610. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Zamberi, N.R.; Abu, N.; Mohammad, N.E.; Rahman, H.S.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Characterization and toxicity of citral incorporated with nanostructured lipid carrier. PeerJ 2018, 6, 3916. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Citral, CID=638011. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Citral (accessed on 24 May 2019).
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) for Poorly Water-Soluble Talinolol: Preparation, in vitro and in vivo Assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Qarni, H.; Alanazi, F.K. Development of oral solid self-emulsifying lipid formulations of risperidone with improved in vitro dissolution and digestion. Eur. J. Pharm. Biopharm. 2017, 114, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.S.; Petersen, K.B.; Mullertz, A. Bioavailability of probucol from lipid and surfactant-based formulations in minipigs: Influence of droplet size and dietary state. Eur. J. Pharm. Biopharm. 2008, 69, 553–562. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Chow, A.H.; Ren, K.; Gong, T.; Zhang, Z.; Zheng, Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int. J. Pharm. 2010, 383, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Saritha, D.; Penjuri, S.C.B.; Nagaraju, R. Formulation and evaluation of self emulsifying drug delivery system (SEDDS) of Indomethacin. Int. J. Res. Pharm. Sci. 2014, 4, 17–23. [Google Scholar]
- Czajkowska-Kośnik, A.; Szekalska, M.; Amelian, A.; Szymańska, E.; Winnicka, K. Development and Evaluation of Liquid and Solid Self-Emulsifying Drug Delivery Systems for Atorvastatin. Molecules 2015, 20, 21010–21022. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; González, C.; Maestro, A.; Solè, I.; Pey, C.M.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Tripathi, S.; Varun, K.; Kaushik, T.; Sanyog, J. Triple antioxidant SNEDDS formulation with enhanced oral bioavailability: Implication of chemoprevention of breast cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [PubMed]
- Hussein, A.A. Preparation and Evaluation of Liquid and Solid Self-Micro Emulsifying Drug Delivery System of Mebendazole. Iraqi. J. Pharm. Sci. 2014, 23, 89–100. [Google Scholar]
- Nazzal, S.; Smalyukh, I.I.; Lavrentovich, O.D.; Khan, M.A. Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: Mechanism and progress of emulsion formation. Int. J. Pharm. 2002, 235, 247–265. [Google Scholar] [CrossRef]
- Date, A.A.; Desai, N.; Dixit, R.; Nagasenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, B.J.; Sreelakshmi, K. Design and Evaluation of Self-Nanoemulsifying Drug Delivery System of Flutamide. J. Young Pharm. 2011, 3, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Eltobshi, A.A.; Mohamed, E.A.; Abdelghani, G.M.; Nouh, A.T. Self-nanoemulsifying drug-delivery systems for potentiated anti-inflammatory activity of diacerein. Int. J. Nanomed. 2018, 13, 6585–6602. [Google Scholar] [CrossRef]
- Patel, A.; Shelat, P.; Lalwani, A. Development and optimization of solid self-nanoemulsifyingdrug delivery system (S-SNEDDS) using Scheffe’s design for improvement of oral bioavailability of nelfinavir mesylate. Drug Deliv. Transl. Res. 2014, 4, 171–186. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Arora, R.; Aggarwal, G. Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharm. 2012, 62, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.K.; Sharma, K.; Kandaswamy, M.; Rajagopal, S.; Mullangi, R. Formulation development of an albendazole self-emulsifying drug delivery system (SEDDS) with enhanced systemic exposure. Acta Pharm. 2012, 62, 563–580. [Google Scholar] [PubMed]
- Obitte, N.C.; Ofokansi, K.C.; Kenechukwu, F.C. Development and Evaluation of Novel Self-Nanoemulsifying Drug Delivery Systems Based on a Homolipid from Capra hircus and Its Admixtures with Melon Oil for the Delivery of Indomethacin. J. Pharm. 2014, 2014, 340486. [Google Scholar]
- Chime, S.A.; Kenechuckwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. Appl. Nanotechnol. Drug Deliv. 2016. [Google Scholar] [CrossRef]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D. A study on factors influencing the droplet size in nonionic oil-in-water microemulsions. Int. J. Pharm. 1992, 88, 417–422. [Google Scholar] [CrossRef]
- Raval, C.; Joshi, N.; Patel, J.; Upadhyay, U. Enhanced oral bioavailability of olmesartan by using novel solid self-emulsifying drug delivery system. Int. J. Adv. Pharm. 2012, 2, 82–92. [Google Scholar]
- Bernardi, D.S.; Pereira, T.A.; Maciel, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; How, C.W.; Abdul, A.B.; Zeenathul, N.A.; Othman, H.H.; Saeed, M.I.; Yeap, S.W. Zerumbone-loaded nanostructured lipid carriers: Preparation, characterization, and antileukemic effect. Int. J. Nanomed. 2013, 8, 2769–2781. [Google Scholar] [CrossRef]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as Vehicles for Transdermal Delivery of Aceclofenac. AAAPS PharmSciTech 2007, 8, 104. [Google Scholar] [CrossRef]
- Badran, M. Formulation and in vitro evaluation of flufenamic acid loaded deformable liposomes for improved skin delivery. Dig. J. Nanomater. Biostruct. 2014, 9, 83–91. [Google Scholar]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Gupta, P.; Jordan, C.T.; Mitov, M.I.; Butterfield, D.A.; Hilt, J.Z.; Dziubla, T.D. Controlled Curcumin Release via Conjugation into PBAE Nanogels Enhances Mitochondrial Protection against Oxidative Stress. Int. J. Pharm. 2016, 511, 1012–1021. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic Approach for the Formulation and Optimization of Solid Lipid Nanoparticles of Efavirenz by High Pressure Homogenization Using Design of Experiments for Brain Targeting and Enhanced Bioavailability. BioMed Res. Int. 2017, 2017, 5984014. [Google Scholar] [CrossRef]
- Abd-Elhakeem, E.; Teaima, M.H.M.; Abdelbary, G.A.; Mahrouk, G.M.E. Bioavailability enhanced clopidogrel-loaded solid SNEDDS: Development and in-vitro/in-vivo characterization. J. Drug Deliv. Sci. Technol. 2019, 49, 603–614. [Google Scholar] [CrossRef]
- Vasquez-Meija, C.M. Effect of Carvacrol-Loaded Nanoemulsionson a Bioluminescent Strain of Escherichia coli O157:H7. Master’s Thesis, Purdue University, West Lafayette, Indiana, 2014. [Google Scholar]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization, and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Burgess, D.J. Colloids and colloid drug delivery system. In Encyclopaedia of Pharmaceutical Technology, 3rd ed.; Informa Healthcare: London, UK, 2006; pp. 636–647. [Google Scholar]
- Ariff, S.A.Y.; Yusoff, K.; Masarudin, M.J. Encapsulation of miRNA in chitosan nanoparticles as a candidate for an anti-metastatic agent in cancer therapy. Malays. Appl. Biol. 2017, 46, 165–170. [Google Scholar]
- Newton, J.E.; Preece, J.A.; Pollet, B.G. Control of nanoparticle aggregation in PEMFCs using surfactant. Int. J. Low-Carbon Technol. 2011, 7, 38–43. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- How, C.W.; Rasedee, A.; Manickam, S.; Rosli, R. Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: Characterization, stability assessment and cytotoxicity. Colloids Surf. B Biointerfaces 2013, 112, 393–399. [Google Scholar] [CrossRef]
- Rahman, H.S.; Rasedee, A.; Othman, H.H.; Chartr and, M.S.; Namvar, F.; Yeap, S.K.; How, C.W. Acute Toxicity Study of Zerumbone-Loaded Nanostructured Lipid Carrier on BALB/c Mice Model. BioMed Res. Int. 2014, 2014, 563930. [Google Scholar] [CrossRef]
- Shah, N.V.; Seth, A.K.; Balaraman, R.; Aundhia, C.J.; Maheshwari, R.A.; Parmar, G.R. Nanostructured lipid carriers for oral bioavailability enhancement of raloxifene: Design and in vivo study. J. Adv. Res. 2016, 7, 423–434. [Google Scholar] [CrossRef]
- Alam, P.N.; Husin, H.; Asnawi, T.M.; Salamun, A. Extraction of citral oil from lemongrass (Cymbopogon citratus) by steam-water distillation technique. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012022. [Google Scholar] [CrossRef]




| SNEDDS | Grade * | Sedimentation |
|---|---|---|
| F1 | A | No |
| F2 | B | Slight |
| F3 | B | Yes |
| F4 | B | Yes |
| F5 | B | Yes |
| F6 | B | Yes |
| F7 | B | Yes |
| F8 | B | Slight |
| F9 | B | Slight |
| F10 | A | Slight |
| F11 | E | No |
| F12 | C | No |
| F13 | B | Yes |
| F14 | C | Yes |
| F15 | A | Slight |
| F16 | B | Yes |
| F17 | B | No |
| F18 | E | No |
| F19 | B | Slight |
| F20 | B | No |
| F21 | B | No |
| F22 | D | Yes |
| F23 | D | Yes |
| F24 | D | Yes |
| F25 | B | No |
| F26 | D | No |
| F27 | B | No |
| Characterization | F1 | F10 | F15 |
|---|---|---|---|
| Particle size | 17.10 ± 0.367 nm | 94.31 ± 0.251 nm | 831.33 ± 18.548 nm |
| Polydispersity index | 0.24 ± 0.003 | 0.08 ± 0.007 | 0.73 ± 0.003 |
| Zeta potential | −0.73 ± 0.249 mV | −1.03 ± 0.092 mV | −2.80 ± 0.056 mV |
| Characterization | 0 Day | 6-Months after Preparation |
|---|---|---|
| Particle size | 17.10 ± 0.367 nm | 44.25 ± 0.102 nm |
| Polydispersity index | 0.24 ± 0.003 | 0.38 ± 0.001 |
| Zeta potential | −0.73 ± 0.249 mV | −1.95 ± 0.082 mV |
| Characterization | Value |
|---|---|
| Particle size | 16.86 ± 0.15 nm |
| Polydispersity index | 0.23 ± 0.01 |
| Zeta potential | 0.58 ± 0.19 mV |
| CIT-SNEDDS release rate | 79.25% |
| Formulation | Zero Order R2 | First Order R2 | Higuchi R2 | Hixson Crowell R2 | Korsemeyer-Peppas R2 (n) |
|---|---|---|---|---|---|
| Citral | 0.9818 | 0.9380 | 0.8239 | 0.9814 | 0.9981 (1.311) |
| CIT-SNEDDS | 0.9930 | 0.8418 | 0.9417 | 0.9183 | 0.9980 (0.7506) |
| Cell Line | Time | IC50 (µg/mL) | ||
|---|---|---|---|---|
| CIT-SNEDDS | Citral | SNEDDS | ||
| HT29 | 24 h | 44.10 ± 0.50 | 28.33 ± 0.33 | 91.80 ± 0.20 |
| 48 h | 36.60 ±0.20 | 22.00 ± 0.00 | 80.30 ± 1.50 | |
| 72 h | 34.10 ± 0.30 | 21.77 ± 0.23 | 63.40 ± 1.00 | |
| SW620 | 24 h | 38.50 ± 0.50 | 31.25 ± 0.75 | 41.33 ± 0.88 |
| 48 h | 23.75 ± 0.25 | 23.30 ± 2.70 | 38.20 ± 0.12 | |
| 72 h | 16.50 ± 0.87 | 22.50 ± 2.50 | 36.33 ± 0.24 | |
| SNEDDS | Oil | Surfactant | Co-Surfactant |
|---|---|---|---|
| F1 | Coconut | Tween 80 | DMSO |
| F2 | Walnut | Tween 80 | DMSO |
| F3 | Almond | Tween 80 | DMSO |
| F4 | Coconut | Tween 40 | DMSO |
| F5 | Walnut | Tween 40 | DMSO |
| F6 | Almond | Tween 40 | DMSO |
| F7 | Coconut | Tween 20 | DMSO |
| F8 | Walnut | Tween 20 | DMSO |
| F9 | Almond | Tween 20 | DMSO |
| F10 | Coconut | Tween 80 | Transcutol |
| F11 | Walnut | Tween 80 | Transcutol |
| F12 | Almond | Tween 80 | Transcutol |
| F13 | Coconut | Tween 40 | Transcutol |
| F14 | Walnut | Tween 40 | Transcutol |
| F15 | Almond | Tween 40 | Transcutol |
| F16 | Coconut | Tween 20 | Transcutol |
| F17 | Walnut | Tween 20 | Transcutol |
| F18 | Almond | Tween 20 | Transcutol |
| F19 | Coconut | Tween 80 | PEG |
| F20 | Walnut | Tween 80 | PEG |
| F21 | Almond | Tween 80 | PEG |
| F22 | Coconut | Tween 40 | PEG |
| F23 | Walnut | Tween 40 | PEG |
| F24 | Almond | Tween 40 | PEG |
| F25 | Coconut | Tween 20 | PEG |
| F26 | Walnut | Tween 20 | PEG |
| F27 | Almond | Tween 20 | PEG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. https://doi.org/10.3390/nano9071028
Mohd Izham MN, Hussin Y, Aziz MNM, Yeap SK, Rahman HS, Masarudin MJ, Mohamad NE, Abdullah R, Alitheen NB. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials. 2019; 9(7):1028. https://doi.org/10.3390/nano9071028
Chicago/Turabian StyleMohd Izham, Mira Nadiah, Yazmin Hussin, Muhammad Nazirul Mubin Aziz, Swee Keong Yeap, Heshu Sulaiman Rahman, Mas Jaffri Masarudin, Nurul Elyani Mohamad, Rasedee Abdullah, and Noorjahan Banu Alitheen. 2019. "Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro" Nanomaterials 9, no. 7: 1028. https://doi.org/10.3390/nano9071028
APA StyleMohd Izham, M. N., Hussin, Y., Aziz, M. N. M., Yeap, S. K., Rahman, H. S., Masarudin, M. J., Mohamad, N. E., Abdullah, R., & Alitheen, N. B. (2019). Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials, 9(7), 1028. https://doi.org/10.3390/nano9071028