Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chlorella vulgaris Culture
2.2. Silver Nanoparticles (Ag NPs) Characterization
2.3. Algal Inhibition Test
2.4. Concentration of Photosynthetic Pigment Chlorophyll a
2.5. Measurement of Viable Cells
2.6. Determination of Chlorophyll a Concentration and Viable Cells Using Silver Nitrate
2.7. Measurement of Reactive Oxygen Species (ROS) Formation
2.8. Transmission Electron Microscopy (TEM) Analyses
2.9. Statistical Analysis
3. Results
3.1. Characterization of Ag NPs
3.2. Concentration of Chlorophyll a
3.3. Viable Cell Concentration
3.4. Determination of Chlorophyll a and Viable Cells Using Silver Nitrate (AgNO3)
3.5. Reactive Oxygen Species (ROS) Production
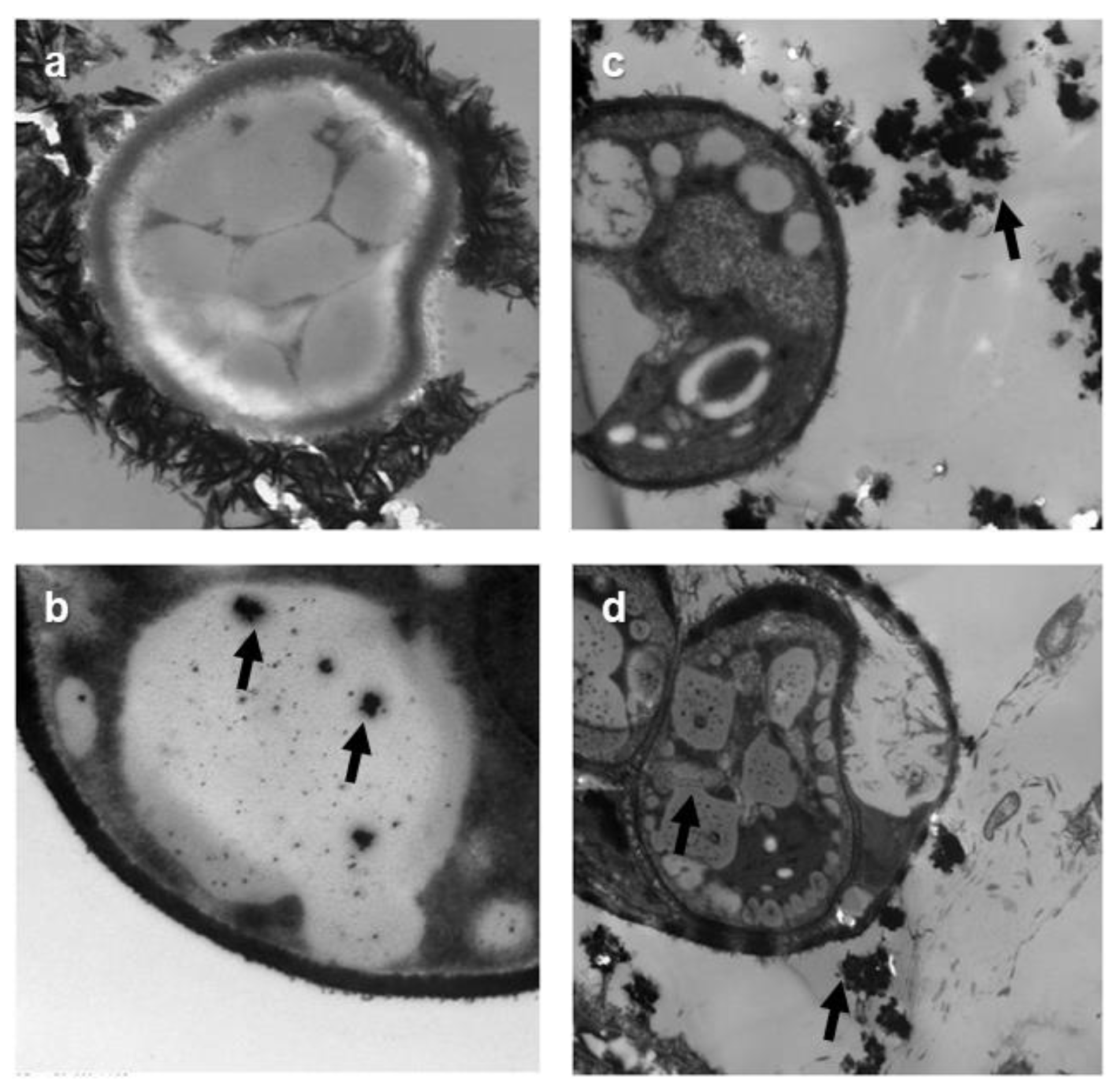
3.6. TEM Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Blinova, I.; Niskanen, J.; Kajankari, P.; Kanarbik, L.; Käkinen, A.; Tenhu, H.; Penttinen, O.P.; Kahru, A. Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environ. Sci. Pollut. Res. Int. 2013, 20, 3456–3463. [Google Scholar] [CrossRef]
- Luoma, S.N. PEN 15-Silver Nanotechnologies and the Environment: Old Problems or New Challenges? Woodrow Wilson International Center for Scholars: Washington, DC, USA, 2008; p. 72. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Navarro, E.; Piccapietra, F.; Wagner, B. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Sambhy, V.; MacBride, M.M.; Peterson, B.R.; Sen, A. Silver bromide nanoparticles/polymer composites: Dual action tunable antimicrobial materials. J. Am. Chem. Soc. 2006, 128, 9798–9808. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticles? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, W. Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ. Sci. Technol. 2010, 44, 7699–7704. [Google Scholar] [CrossRef]
- Bondarenko, O.; Juganson, K.; Ivask, A.; Kasemets, K.; Mortimer, M.; Kahru, A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: A critical review. Arch. Toxicol. 2013, 87, 1181–1200. [Google Scholar] [CrossRef]
- Maynard, A.D.; Aitken, R.J.; Butz, T.; Colvin, V.; Donaldson, K.; Oberdörster, G.; Philbert, M.A.; Ryan, J.; Seaton, A.; Stone, V.; et al. Safe handling of nanotechnology. Nature 2006, 444, 267–269. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Alfaro, S.O.; Torres-Martínez, L.M.; Cho, S.; Lee, S. Silver-TiO2 nanocomposites: Synthesis and harmful algae bloom UV-photoelimination. Appl. Catal. B Environ. 2010, 98, 229–234. [Google Scholar] [CrossRef]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano-micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbuhler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Morales, J.D.M.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological system matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Yin, L.; Ren, N.N.; Xian, L.; Zhao, S.; Li, W.; Gontero, B. The effect of chronic silver nanoparticles on aquatic system in microcosms. Environ. Pollut. 2017, 223, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Hull, M.S.; Bednar, A.J.; Goss, J.D.; Gunter, J.C.; Bouldin, J.L.; Vikesland, P.J.; Steevens, J.A. Fractionating nanosilver: Importance for determining toxicity to aquatic test organisms. Environ. Sci. Technol. 2010, 44, 9571–9577. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bielmyer, G.K.; Bell, R.A.; Klaine, S.J. Effects of ligand-bound silver on Ceriodaphnia dubia. Environ. Toxicol. Chem. 2002, 21, 2204–2208. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Boyle, T.P. The effect of environmental contaminants on aquatic algae. In Algae as Ecological Indicators; Shubert, L.E., Ed.; Academic Press: New York, NY, USA, 1984; pp. 237–256. [Google Scholar]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles under natural freshwater conditions. Environ. Chem. 2014, 12, 138–148. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Bras, S.; Perreault, F.; Popovic, R. Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol. Environ. Saf. 2012, 78, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Polchtchkov, S.; Perreault, F.; Popovic, R. Temperature influence on silver nanoparticles inhibitory effect on Photosystem II photochemistry in two green algae, Chlorella vulagris, and Dunaliella teriolecta. Environ. Sci. Pollut. Res. 2012, 19, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Ksiąźyk, M.; Asztemborska, M.; Stęborowski, R.; Bystrzejewska-Piotrowska, G. Toxic effect of silver and platinum nanoparticles toward the freshwater microalgae Pseudokirchneriella subcapitata. Bull. Environ. Contam. Toxicol. 2015, 94, 554–558. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Dorantes-Aranda, J.J.; Waite, T.D. Silver nanoparticles-algae interactions: Oxidative dissolution, reactive oxygen species generation and synergistic toxic effects. Environ. Sci. Technol. 2012, 46, 8731–8738. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef]
- Sager, T.M.; Porter, D.W.; Robinson, V.A.; Lindsley, W.G.; Schwegler-Berry, D.E.; Castranova, V. Improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology 2007, 1, 118–129. [Google Scholar] [CrossRef]
- Panessa-Warren, B.J.; Maye, M.M.; Warren, J.B.; Crosson, K.M. Single walled carbon nanotube reactivity and cytotoxicity following extended aqueous exposure. Environ. Pollut. 2009, 157, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I.; Pére, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, I.; Dewez, D. Toxicity of superparamegentic iron oxide nanoparticles on green alga Chlorella vulgaris. Biomed. Res. Int. 2013, 647974. [Google Scholar] [CrossRef]
- Angel, B.M.; Batley, G.E.; Jarolimek, C.V.; Rogers, N.J. The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 2013, 93, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, A.D.; Carvalho, R.N.; Valente, A.; Nativo, P.; Gilliland, D.; Garcìa, C.P.; Passarella, R.; Pedroni, V.; Rossi, F.; Lettieri, T. Effects of silver nanoparticles in diatom Thalassiosira pseudonana and cyanobacterium Synechococcus sp. Environ. Sci. Technol. 2012, 46, 11336–11344. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Goss, K.U. Volatilization modeling of two herbicides from soil in a wind tunnel experiment under varying humidity conditions. Environ. Sci. Technol. 2012, 46, 12527–12533. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.C. Determination of Photosynthetic Pigment in Seawater. Monographs on Oceanographic Methodology; UNESCO: Paris, France, 1966; p. 66. [Google Scholar]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Yeste, M.P.; Gatica, J.M.; Moreno-Garrido, I.; Blasco, J. Direct and indirect effects of silver nanoparticles on freshwater and marine microalgae (Chlamydomonas reinhardtii and Phaeodactylum tricornutum). Chemosphere 2017, 179, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H. Physiochemical properties of protein-modified silver nanoparticles in seawater. Int. Nano Lett. 2013, 3, 54. [Google Scholar] [CrossRef]
- Leclerc, S.; Wilkinson, K.J. Bioaccumulation of Nanosilver by Chlamydomonas reinhardtii-Nanoparticle or the Free Ion? Environ. Sci. Technol. 2014, 48, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.I.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Johari, S.A.; Sarkheil, M.; Tayemeh, M.B.; Veisi, S. Influence of salinity on the toxicity of silver nanoparticles (AgNPs) and silver nitrate (AgNO3) in halophilic microalgae, Dunaliella salina. Chemosphere 2018, 209, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reinfelder, J.R.; Change, S. Speciation and Microalgal Bioavialability of Inorganic Silver. Environ. Sci. Technol. 1999, 33, 1860–1863. [Google Scholar] [CrossRef]
- Leonardo, T.; Farhi, E.; Pouget, S.; Motellier, S.; Boisson, A.; Banerjee, D.; Rébeillé, F.; Auwer, C.; Rivasseau, C. Silver Accumulation in the Green Microalga Coccomyxa actinabiotis: Toxicity, in Situ Speciation, and Localization Investigated Using Synchrotron XAS, XRD, and TEM. Environ. Sci. Technol. 2016, 50, 359–367. [Google Scholar] [CrossRef]
- Chang, Y.N.; Zhang, M.Y.; Xia, I.; Zhang, J.; Xing, G.M. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Maeda, S.; Sakaguchi, T. Accumulation and detoxification of toxic elements by algae. In Introduction to Applied Phycology; Akatsuka, I., Ed.; SPB Academic Publishing: The Hage, The Netherlands, 1990. [Google Scholar]
- Troung, L.; Zaikova, T.; Richman, E.K.; Hutchison, J.E.; Tanguay, R.L. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology 2012, 6, 691–699. [Google Scholar] [CrossRef]
- Handy, R.D.; Kammer, F.V.D.; Lead, J.R.; Hassellöv, M.; Owen, R.; Grane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazeem, L.J.; Kuku, G.; Dewailly, E.; Slomianny, C.; Barras, A.; Hamdi, A.; Boukherroub, R.; Culha, M.; Bououdina, M. Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris. Nanomaterials 2019, 9, 914. https://doi.org/10.3390/nano9070914
Hazeem LJ, Kuku G, Dewailly E, Slomianny C, Barras A, Hamdi A, Boukherroub R, Culha M, Bououdina M. Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris. Nanomaterials. 2019; 9(7):914. https://doi.org/10.3390/nano9070914
Chicago/Turabian StyleHazeem, Layla J., Gamze Kuku, Etienne Dewailly, Christian Slomianny, Alexandre Barras, Abderrahmane Hamdi, Rabah Boukherroub, Mustafa Culha, and Mohamed Bououdina. 2019. "Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris" Nanomaterials 9, no. 7: 914. https://doi.org/10.3390/nano9070914
APA StyleHazeem, L. J., Kuku, G., Dewailly, E., Slomianny, C., Barras, A., Hamdi, A., Boukherroub, R., Culha, M., & Bououdina, M. (2019). Toxicity Effect of Silver Nanoparticles on Photosynthetic Pigment Content, Growth, ROS Production and Ultrastructural Changes of Microalgae Chlorella vulgaris. Nanomaterials, 9(7), 914. https://doi.org/10.3390/nano9070914





