Dopant-Free Hole Transport Materials with a Long Alkyl Chain for Stable Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Device Characterization
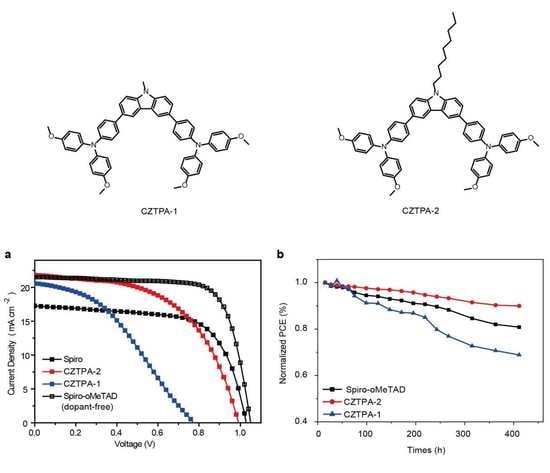
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Michael, M.; Lee, J.L.T.T. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [Green Version]
- Kim, H.; Lee, C.; Im, J.; Lee, K.; Moehl, T.; Marchioro, A.; Moon, S.; Humphry-Baker, R.; Yum, J.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laura Calil, S.K.M.G. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.M.; Schenk, K.; Abate, A.; Giordano, F.; Correa Baena, J.; et al. Efficient luminescent solar cells based on tailored mixed-cation perovskites. Sci. Adv. 2016, 2, 1501170–1501176. [Google Scholar] [CrossRef] [PubMed]
- NREL Best Research Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-190416.pdf (accessed on 27 June 2019).
- Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissortel, F.; Salbeck, J.; Spretzer, H.; Gratzel, M. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. o-Methoxy Substituents in Spiro-OMeTAD for Efficient Inorganic—Organic Hybrid Perovskite Solar Cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef]
- Gatti, T.; Menna, E.; Meneghetti, M.; Maggini, M.; Petrozza, A.; Lamberti, F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy 2017, 41, 84–100. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 1808843. [Google Scholar] [CrossRef]
- Castro, E.; Murillo, J.; Fernandez-Delgado, O.; Echegoyen, L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liang, P.; Williams, S.T.; Cho, N.; Chueh, C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K.Y. High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Gr tzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.; Chang, J.A.; Lee, Y.H.; Kim, H.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Edri, E.; Kirmayer, S.; Cahen, D.; Hodes, G. High Open-Circuit Voltage Solar Cells Based on Organic?—CInorganic Lead Bromide Perovskite. J. Phys. Chem. Lett. 2013, 4, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Cabau, L.; Garcia-Benito, I.; Molina-Ontoria, A.; Montcada, N.F.; Martin, N.; Vidal-Ferran, A.; Palomares, E. Diarylamino-substituted tetraarylethene (TAE) as an efficient and robust hole transport material for 11% methyl ammonium lead iodide perovskite solar cells. Chem. Commun. 2015, 51, 13980–13982. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Fu, K.; Hagfeldt, A.; Gr tzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. A Simple 3,4-Ethylenedioxythiophene Based Hole-Transporting Material for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 4085–4088. [Google Scholar] [CrossRef]
- Li, H.; Fu, K.; Boix, P.P.; Wong, L.H.; Hagfeldt, A.; Gr tzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. Hole-Transporting Small Molecules Based on Thiophene Cores for High Efficiency Perovskite Solar Cells. ChemSusChem 2014, 7, 3420–3425. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupr, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Qin, R.; Li, W.; Li, C.; Du, C.; Veit, C.; Schleiermacher, H.; Andersson, M.; Bo, Z.; Liu, Z.; Ingana s, O.; et al. A Planar Copolymer for High Efficiency Polymer Solar Cells. J. Am. Chem. Soc. 2009, 131, 14612–14613. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s: Structure—Property Relationships. Acc. Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.; Michaud, A.; Leclerc, M. A Low-Bandgap Poly(2,7-Carbazole) Derivative for Use in High-Performance Solar Cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Zhu, X.D.; Ma, X.J.; Wang, Y.K.; Li, Y.; Gao, C.H.; Wang, Z.K.; Jiang, Z.Q.; Liao, L.S. Hole-Transporting Materials Incorporating Carbazole into Spiro-Core for Highly Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2018, 29, 1807094. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Zheng, X.; Zhang, Q.; Li, Z.; Hao, Y.; Fang, G. Low-Cost Carbazole-Based Hole-Transport Material for Highly Efficient Perovskite Solar Cells. ChemSusChem 2017, 10, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sheibani, E.; Liu, P.; Zhang, J.; Tian, H.; Vlachopoulos, N.; Boschloo, G.; Kloo, L.; Hagfeldt, A.; Sun, L. Carbazole-Based Hole-Transport Materials for Efficient Solid-State Dye-Sensitized Solar Cells and Perovskite Solar Cells. Adv. Mater. 2014, 26, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Paek, S.; Giordano, F.; Correa-Baena, J.P.; Saliba, M.; Gao, P.; Matsui, T.; Ko, J.; Zakeeruddin, S.M.; Dahmen, K.H.; et al. Silolothiophene-linked triphenylamines as stable hole transporting materials for high efficiency perovskite solar cells. Energy Environ. Sci. 2015, 8, 2946–2953. [Google Scholar] [CrossRef]
- Yin, X.; Guan, L.; Yu, J.; Zhao, D.; Wang, C.; Shrestha, N.; Han, Y.; An, Q.; Zhou, J.; Zhou, B.; et al. One-step facile synthesis of a simple carbazole-cored hole transport material for high-performance perovskite solar cells. Nano Energy 2017, 40, 163–169. [Google Scholar] [CrossRef]
- Zimmermann, I.; Urieta-Mora, J.; Gratia, P.; Arag, J.; Grancini, G.; Molina-Ontoria, A.N.; Ort, E.; Mart n, N.; Nazeeruddin, M.K. High-Efficiency Perovskite Solar Cells Using Molecularly Engineered, Thiophene-Rich, Hole-Transporting Materials: Influence of Alkyl Chain Length on Power Conversion Efficiency. Adv. Funct. Mater. 2017, 7, 1601674. [Google Scholar] [CrossRef]
- Wang, Y.; Su, T.; Tsai, H.; Wei, T.; Chi, Y. Spiro-Phenylpyrazole/Fluorene as Hole-Transporting Material for Perovskite Solar Cells. Sci. Rep. 2017, 7, 7859–7867. [Google Scholar] [CrossRef]






| λmax Sol (a) (nm) | λmax Film (b) (nm) | λonset (nm) | Egopt (c) (eV) | EHOMO (eV) | ELUMO (d) (eV) | |
|---|---|---|---|---|---|---|
| CZTPA-1 | 335 | 337 | 409 | 3.03 | −4.89 | −1.86 |
| CZTPA-2 | 327 | 339 | 416 | 2.98 | −4.83 | −1.85 |
| Spiro-OMeTAD | 386 | 390 | 428 | 2.90 | −4.64 | −1.74 |
| VOC (V) | JSC (mA·cm−2) | FF (%) | PCE (%) | PCE Ave (%) | |
|---|---|---|---|---|---|
| Spiro-OMeTAD (dopant-free) | 1.02 | 17.26 | 66.14 | 11.74 | 10.02% ± 0.98 |
| CZTPA-2 | 0.99 | 21.80 | 54.59 | 11.79 | 10.15% ± 0.90 |
| CZTPA-1 | 0.77 | 20.58 | 38.01 | 6.05 | 5.27% ± 0.57 |
| Spiro-OMeTAD | 1.05 | 21.51 | 74.24 | 16.77 | 15.65% ± 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Chen, H.; Niu, T.; Wang, S.; Guo, X.; Wang, H. Dopant-Free Hole Transport Materials with a Long Alkyl Chain for Stable Perovskite Solar Cells. Nanomaterials 2019, 9, 935. https://doi.org/10.3390/nano9070935
Wang K, Chen H, Niu T, Wang S, Guo X, Wang H. Dopant-Free Hole Transport Materials with a Long Alkyl Chain for Stable Perovskite Solar Cells. Nanomaterials. 2019; 9(7):935. https://doi.org/10.3390/nano9070935
Chicago/Turabian StyleWang, Kai, Haoran Chen, Tingting Niu, Shan Wang, Xiao Guo, and Hong Wang. 2019. "Dopant-Free Hole Transport Materials with a Long Alkyl Chain for Stable Perovskite Solar Cells" Nanomaterials 9, no. 7: 935. https://doi.org/10.3390/nano9070935