Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions
Abstract
:1. Introduction
2. Biodegradable and Biocompatible Polymers
2.1. Insulated Polymers
2.2. Conductive and Semiconductive Polymers
3. Applications of Nanocomposites for Electronics
3.1. Substrates
3.2. Conductors and Semiconductors
3.3. Dielectrics
4. Electronics Packaging
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Uhrich, K.E. Biodegradable and biocompatible polymers for electronic applications: A review. J. Bioact. Compat. Polym. 2019, 34, 3–15. [Google Scholar] [CrossRef]
- Ogunseitan, O.A.; Schoenung, J.M.; Saphores, J.-D.M.; Shapiro, A.A. The Electronics Revolution: From E-Wonderland to E-Wasteland. Science 2009, 326, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.N.; Zhu, C.P.; Li, Y.; Lei, X.F.; Zhang, W.; Xiao, J.L. Rehealable, fully recyclable, and malleable electronic skin enabled by dynamic covalent thermoset nanocomposite. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.-C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.-C.; Shao, L.; Cheng, K.-T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, K.K.; Wang, Z.; Dai, J.; Carter, M.; Hu, L. Transient Electronics: Materials and Devices. Chem. Mater. 2016, 28, 3527–3539. [Google Scholar] [CrossRef]
- Tafazoli, S.; Rafiemanzelat, F.; Hassanzadeh, F.; Rostami, M. Synthesis and characterization of novel biodegradable water dispersed poly(ether-urethane)s and their MWCNT-AS nanocomposites functionalized with aspartic acid as dispersing agent. Iran. Polym. J. 2018, 27, 755–774. [Google Scholar] [CrossRef]
- Salehpour, S.; Jonoobi, M.; Ahmadzadeh, M.; Siracusa, V.; Rafieian, F.; Oksman, K. Biodegradation and ecotoxicological impact of cellulose nanocomposites in municipal solid waste composting. Int. J. Biol. Macromol. 2018, 111, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Rao, K.S.V.K.; Haider, A.; Han, S.S. Biodegradable Tragacanth Gum Based Silver Nanocomposite Hydrogels and Their Antibacterial Evaluation. J. Polym. Environ. 2018, 26, 778–788. [Google Scholar] [CrossRef]
- Gao, X.; Huang, L.; Wang, B.; Xu, D.; Zhong, J.; Hu, Z.; Zhang, L.; Zhou, J. Natural Materials Assembled, Biodegradable, and Transparent Paper-Based Electret Nanogenerator. ACS Appl. Mater. Interfaces 2016, 8, 35587–35592. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Nguyen, L.T.H.; Surendran, A.; Tan, B.Y.; Ng, K.W.; Leong, W.L. Human Hair Keratin for Biocompatible Flexible and Transient Electronic Devices. ACS Appl. Mater. Interfaces 2017, 9, 43004–43012. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lim, Y.W.; Im, H.G.; Jeong, S.; Ji, S.; Kim, Y.H.; Choi, G.M.; Park, J.U.; Lee, J.Y.; Jin, J.; et al. Bioinspired Transparent Laminated Composite Film for Flexible Green Optoelectronics. ACS Appl. Mater. Interfaces 2017, 9, 24161–24168. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, S.; Heller, M.J. An Implantable Transparent Conductive Film with Water Resistance and Ultrabendability for Electronic Devices. ACS Appl. Mater. Interfaces 2017, 9, 42302–42312. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Pinto, V.C.; Costa-Almeida, R.; Rodrigues, I.; Guardao, L.; Soares, R.; Guedes, R.M. Exploring the in vitro and in vivo compatibility of PLA, PLA/GNP and PLA/CNT-COOH biodegradable nanocomposites: Prospects for tendon and ligament applications. J. Biomed. Mater. Res. Part A 2017, 105, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kaream, S.A.; Abd Elsamie, G.H.; Abd-Alkareem, A.S. Sono-photodynamic modality for cancer treatment using biodegradable bio-conjugated sonnelux nanocomposite in tumor-bearing mice: Activated cancer therapy using light and ultrasound. Biochem. Biophys. Res. Commun. 2018, 503, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Guo, Y.; Niu, W.; Chen, M.; Xue, Y.M.; Ge, J.; Ma, P.X.; Lei, B. Biodegradable Multifunctional Bioactive Glass-Based Nanocomposite Elastomers with Controlled Biomineralization Activity, Real-Time Bioimaging Tracking, and Decreased Inflammatory Response. ACS Appl. Mater. Interfaces 2018, 10, 17722–17731. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic Acid Based Nanocomposites: Promising Safe and Biodegradable Materials in Biomedical Field. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable polymeric materials in degradable electronic devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Chivrac, F.; Pollet, E.; Averous, L. Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater. Sci. Eng. R Rep. 2009, 67, 1–17. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Sun, J.Y.; Shen, J.J.; Chen, S.K.; Cooper, M.A.; Fu, H.B.; Wu, D.M.; Yang, Z.G. Nanofiller Reinforced Biodegradable PLA/PHA Composites: Current Status and Future Trends. Polymers 2018, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bao, Z. Biomaterials-based organic electronic devices. Polym. Int. 2010, 59, 563–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, F.; Klauk, H.; Halik, M.; Zschieschang, U.; Schmid, G.; Dehm, C. Organic electronics on paper. Appl. Phys. Lett. 2004, 84, 2673–2675. [Google Scholar] [CrossRef]
- Bollström, R.; Määttänen, A.; Tobjörk, D.; Ihalainen, P.; Kaihovirta, N.; Österbacka, R.; Peltonen, J.; Toivakka, M. A multilayer coated fiber-based substrate suitable for printed functionality. Org. Electron. 2009, 10, 1020–1023. [Google Scholar] [CrossRef]
- Petritz, A.; Wolfberger, A.; Fian, A.; Griesser, T.; Irimia-Vladu, M.; Stadlober, B. Cellulose-Derivative-Based Gate Dielectric for High-Performance Organic Complementary Inverters. Adv. Mater. 2015, 27, 7645–7656. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009, 95, 1–3. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Wang, C.H.; Hsieh, C.Y.; Hwang, J.C. Flexible Organic Thin-Film Transistors with Silk Fibroin as the Gate Dielectric. Adv. Mater. 2011, 23, 1630–1634. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Glowacki, E.D.; Schwabegger, G.; Leonat, L.; Akpinar, H.Z.; Sitter, H.; Bauer, S.; Sariciftci, N.S. Natural resin shellac as a substrate and a dielectric layer for organic field-effect transistors. Green Chem. 2013, 15, 1473–1476. [Google Scholar] [CrossRef]
- Baek, S.W.; Ha, J.W.; Yoon, M.; Hwang, D.H.; Lee, J. Shellac Films as a Natural Dielectric Layer for Enhanced Electron Transport in Polymer Field-Effect Transistors. ACS Appl. Mater. Interfaces 2018, 10, 18948–18955. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cebe, P.; Weiss, A.S.; Omenetto, F.; Kaplan, D.L. Protein-based composite materials. Mater. Today 2012, 15, 208–215. [Google Scholar]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwodiauer, R.; Mumyatov, A.; Fergus, J.W.; et al. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Ning, N.; Wang, Z.; Yao, Y.; Zhang, L.; Tian, M. Enhanced electromechanical performance of bio-based gelatin/glycerin dielectric elastomer by cellulose nanocrystals. Carbohyd. Polym. 2015, 130, 262–267. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, J.; Qian, C.; Kong, L.-a.; Jiang, J.; Yang, J.; Li, H.; Gao, Y. Solution-processed natural gelatin was used as a gate dielectric for the fabrication of oxide field-effect transistors. Org. Electron. 2016, 38, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.M.; Zhang, D.Y.; Wang, X.L.; Yu, X.G.; Yu, J.S. Biocompatible and degradable gelatin dielectric based low-operating voltage organic transistors for ultra-high sensitivity NH3 detection. Appl. Phys. Lett. 2018, 113, 1–4. [Google Scholar] [CrossRef]
- Li, Y.; Neoh, K.G.; Kang, E.T. Controlled Release of Heparin from Polypyrrole-Poly(vinyl alcohol) Assembly by Electrical Stimulation. J. Biomed. Mater. Res. Part A 2005, 73A, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: a review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef]
- Afsharimani, N.; Nysten, B. Hybrid gate dielectrics: A comparative study between polyvinyl alcohol/SiO2 nanocomposite and pure polyvinyl alcohol thin-film transistors. Bull. Mater. Sci. 2019, 42, 26. [Google Scholar] [CrossRef]
- Nawaz, A.; Hummelgen, I.A. Poly(vinyl alcohol) gate dielectric in organic field-effect transistors. J. Mater. Sci. Mater. Electron. 2019, 30, 5299–5326. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Chew, D.J.; Minev, I.R.; Fawcett, J.W.; Lacour, S.P. Concurrent recordings of bladder afferents from multiple nerves using a microfabricated PDMS microchannel electrode array. Lab Chip 2012, 12, 2540–2551. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chan-Park, M.B.; Li, C.M. Adhesive-Free Transfer of Gold Patterns to PDMS-Based Nanocomposite Dielectric for Printed High-Performance Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2011, 3, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, H.; He, X.; Liu, G.; Xi, Y.; Shi, H.; Hu, C. Enhancing Performance of Triboelectric Nanogenerator by Filling High Dielectric Nanoparticles into Sponge PDMS Film. ACS Appl. Mater. Interfaces 2016, 8, 736–744. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Mu, X.; Wen, Q.; Wen, Z.; Yang, J.; Hu, C.; Shi, H. Flexible and transparent triboelectric nanogenerator based on high performance well-ordered porous PDMS dielectric film. Nano Res. 2016, 9, 3714–3724. [Google Scholar] [CrossRef]
- Rajitha, G.; Dash, R.K. Optically transparent and high dielectric constant reduced graphene oxide (RGO)-PDMS based flexible composite for wearable and flexible sensors. Sens. Actuator A Phys. 2018, 277, 26–34. [Google Scholar] [CrossRef]
- Shi, X.W.; Dai, X.; Cao, Y.; Li, J.W.; Huo, C.G.; Wang, X.L. Degradable Poly(lactic acid)/Metal-Organic Framework Nanocomposites Exhibiting Good Mechanical, Flame Retardant, and Dielectric Properties for the Fabrication of Disposable Electronics. Ind. Eng. Chem. Res. 2017, 56, 3887–3894. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.C.; Wang, X.X.; Meng, J.P.; Zou, Y.; Noreen, S.; Zhao, L.M.; Liu, Z.; Ouyang, H.; Tan, P.C.; et al. Fully Bioabsorbable Capacitor as an Energy Storage Unit for Implantable Medical Electronics. Adv. Sci. 2019, 6, 1–9. [Google Scholar]
- Wu, X.H.; Ma, Y.; Zhang, G.Q.; Chu, Y.L.; Du, J.; Zhang, Y.; Li, Z.; Duan, Y.R.; Fan, Z.Y.; Huang, J. Thermally Stable, Biocompatible, and Flexible Organic Field-Effect Transistors and Their Application in Temperature Sensing Arrays for Artificial Skin. Adv. Funct. Mater. 2015, 25, 2138–2146. [Google Scholar] [CrossRef]
- Meriakri, V.V.; Parkhomenko, M.P.; Kalenov, D.S.; Fedoseev, N.A.; Sh, Z. Dielectric properties of biocompatible and biodegradated poly-caprolactone, polylactide and its nanocomposites in the millimeter wave range. Elektromagn. Volny Elektron. Sist. 2012, 17, 30–33. [Google Scholar]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagné, S.; Bao, Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bao, Z. Organic Thin-Film Transistors Fabricated on Resorbable Biomaterial Substrates. Adv. Mater. 2010, 22, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.-K.; Chang, H.-P.; Guo, Q.; Koo, J.; Wu, C.-I.; Rogers, J.A. Biodegradable electronic systems in 3D, heterogeneously integrated formats. Adv. Mater. 2018, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bidez, P.R.; Li, S.; MacDiarmid, A.G.; Venancio, E.C.; Wei, Y.; Lelkes, P.I. Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 199–212. [Google Scholar] [CrossRef] [Green Version]
- George, P.M.; Lyckman, A.W.; Lavan, D.A.; Anita, H.; Yuika, L.; Rupali, A.; Chris, T.; Alexander, P.M.; Robert, L.; Mriganka, S. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, H.; Li, Y.; Zhang, X. Biodegradable Transparent Substrate Based on Edible Starch-Chitosan Embedded with Nature-Inspired Three-Dimensionally Interconnected Conductive Nanocomposites for Wearable Green Electronics. ACS Appl. Mater. Interfaces 2018, 10, 23037–23047. [Google Scholar] [CrossRef]
- Fortunato, E.; Correia, N.; Barquinha, P.; Pereira, L.; Goncalves, G.; Martins, R. High-Performance Flexible Hybrid Field-Effect Transistors Based on Cellulose Fiber Paper. IEEE Electron Device Lett. 2008, 29, 988–990. [Google Scholar] [CrossRef] [Green Version]
- Zschieschang, U.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Klauk, H. Organic electronics on banknotes. Adv. Mater. 2011, 23, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ren, X.; Wang, Z.; Wang, X.; Roberts, R.C.; Chan, P.K. High performance organic transistor active-matrix driver developed on paper substrate. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Leonat, L.; White, M.S.; Głowacki, E.D.; Scharber, M.C.; Zillger, T.; Rühling, J.; Hübler, A.; Sariciftci, N.S. 4% efficient polymer solar cells on paper substrates. J. Phys. Chem. C 2014, 118, 16813–16817. [Google Scholar] [CrossRef]
- Barr, M.C.; Rowehl, J.A.; Lunt, R.R.; Xu, J.; Wang, A.; Boyce, C.M.; Im, S.G.; Bulović, V.; Gleason, K.K. Direct monolithic integration of organic photovoltaic circuits on unmodified paper. Adv. Mater. 2011, 23, 3500–3505. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, W.; Gong, A.; Gong, T.; Ma, D.; Wan, J.; Dai, J.; Munday, J.N.; He, J.-H.; Hu, L.; et al. A highly sensitive, highly transparent, gel-gated MoS2 phototransistor on biodegradable nanopaper. Nanoscale 2016, 8, 14237–14242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petritz, A.; Wolfberger, A.; Fian, A.; Irimia-Vladu, M.; Haase, A.; Gold, H.; Rothlander, T.; Griesser, T.; Stadlober, B. Cellulose as biodegradable high-k dielectric layer in organic complementary inverters. Appl. Phys. Lett. 2013, 103, 1–5. [Google Scholar] [CrossRef]
- Cunha, I.; Barras, R.; Grey, P.; Gaspar, D.; Fortunato, E.; Martins, R.; Pereira, L. Reusable Cellulose-Based Hydrogel Sticker Film Applied as Gate Dielectric in Paper Electrolyte-Gated Transistors. Adv. Funct. Mater. 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Dai, S.L.; Chu, Y.L.; Liu, D.P.; Cao, F.; Wu, X.H.; Zhou, J.C.; Zhou, B.L.; Chen, Y.T.; Huang, J. Intrinsically ionic conductive cellulose nanopapers applied as all solid dielectrics for low voltage organic transistors. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, C.; Ghosh, S.K.; Kundu, S. Silk fibroin film from non-mulberry tropical tasar silkworms: A novel substrate for in vitro fibroblast culture. Acta Biomater. 2009, 5, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.-Y.; Chen, X. Silk fibroin for flexible electronic devices. Adv. Mater. 2016, 28, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Hwang, S.-W.; Marelli, B.; An, B.; Moreau, J.E.; Yang, M.; Brenckle, M.A.; Kim, S.; Kaplan, D.L.; Rogers, J.A. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 2014, 111, 17385–17389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, H.; Brenckle, M.A.; Yang, M.; Zhang, J.; Liu, M.; Siebert, S.M.; Averitt, R.D.; Mannoor, M.S.; McAlpine, M.C.; Rogers, J.A. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 2012, 24, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.C.; Huang, Y.H.; Kuo, C.C.; Cho, C.J.; Rwei, S.P.; Jia, Q.; Ding, Y.S.; Chen, Y.G.; Borsali, R. Thermally deposited silk fibroin as the gate dielectric layer in organic thin-film transistors based on conjugated polymer. React. Funct. Polym. 2018, 131, 368–377. [Google Scholar] [CrossRef]
- Tsai, L.-S.; Hwang, J.-C.; Lee, C.-Y.; Lin, Y.-T.; Tsai, C.-L.; Chang, T.-H.; Chueh, Y.-L.; Meng, H.-F. Solution-based silk fibroin dielectric in n-type C-60 organic field-effect transistors: Mobility enhancement by the pentacene interlayer. Appl. Phys. Lett. 2013, 103, 1–4. [Google Scholar] [CrossRef]
- Li, H.-Q.; Yu, J.-S.; Huang, W.; Shi, W.; Huang, J. High performance pentacene organic field-effect transistors consisting of biocompatible PMMA/silk fibroin bilayer dielectric. Chin. Phys. B 2014, 23, 1–4. [Google Scholar] [CrossRef]
- Shi, L.; Xu, X.; Ma, M.; Li, L. High-performance, low-operating voltage, and solution-processable organic field-effect transistor with silk fibroin as the gate dielectric. Appl. Phys. Lett. 2014, 104, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Shi, W.; Yu, X.; Yu, J. Performance improvement of organic field-effect transistor based nitrogen dioxide gas sensor using biocompatible PMMA/silk fibroin bilayer dielectric. J. Mater. Sci.-Mater. Electron. 2015, 26, 7948–7954. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, J.; Lee, S.C.; Cho, S.Y.; Kim, N.R.; Kang, B.; Song, E.; Cho, K.; Jin, H.-J.; Lee, W.H. Critical role of silk fibroin secondary structure on the dielectric performances of organic thin-film transistors. RSC Adv. 2016, 6, 5907–5914. [Google Scholar] [CrossRef]
- Zhuang, X.; Huang, W.; Yang, X.; Han, S.; Li, L.; Yu, J. Biocompatible/Degradable Silk Fibroin:Poly(Vinyl Alcohol)-Blended Dielectric Layer Towards High-Performance Organic Field-Effect Transistor. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwak, H.W.; Park, M.H.; Hwang, J.; Kim, J.W.; Jang, H.W.; Jin, H.-J.; Lee, W.H. Understanding hydroscopic properties of silk fibroin and its use as a gate-dielectric in organic field-effect transistors. Org. Electron. 2018, 59, 213–219. [Google Scholar] [CrossRef]
- Weinberger, H.; Gardner, W.H. Chemical composition of shellac. Ind. Eng. Chem. 1938, 30, 454–458. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Irimia-Vladu, M.; Głowacki, E.D.; Troshin, P.A.; Schwabegger, G.; Leonat, L.; Susarova, D.K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M.A. Indigo-a natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv. Mater. 2012, 24, 375–380. [Google Scholar] [CrossRef]
- Zhuang, J.; Wu, D.-M.; Xu, H.; Huang, Y.; Liu, Y.; Sun, J.-Y. Edge Effect in Hot Embossing and its Influence on Global Pattern Replication of Polymer-Based Microneedles. Int. Polym. Proc. 2019, 34, 231–238. [Google Scholar] [CrossRef]
- Xi, H.; Chen, D.; Lv, L.; Zhong, P.; Lin, Z.; Chang, J.; Wang, H.; Wang, B.; Ma, X.; Zhang, C. High performance transient organic solar cells on biodegradable polyvinyl alcohol composite substrates. RSC Adv. 2017, 7, 52930–52937. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Han, J.; Choi, B.; Lee, Y.; Kim, Y.; Park, J.; Lim, M.; Kang, M.-H.; Kim, D.H.; Kim, D.M.; et al. Three-Dimensional Printed Poly(vinyl alcohol) Substrate with Controlled On-Demand Degradation for Transient Electronics. ACS Nano 2018, 12, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, J.; Hu, W.; Fan, Y.; Sun, J.; He, X.; Xu, H.; Huang, Y.; Wu, D. Fabrication and testing of metal/polymer microstructure heat exchangers based on micro embossed molding method. Microsyst. Technol. 2019, 25, 381–388. [Google Scholar] [CrossRef]
- Jingyao, S.; Daming, W.; Ying, L.; Zhenzhou, Y.; Pengsheng, G. Rapid fabrication of micro structure on polypropylene by plate to plate isothermal hot embossing method. Polym. Eng. Sci. 2018, 58, 952–960. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, J.; Jiang, H.; Huang, Y.; Zheng, X.; Liu, Y.; Wu, D. Thermal dissipation performance of metal-polymer composite heat exchanger with V-shape microgrooves: A numerical and experimental study. Appl. Therm. Eng. 2017, 121, 492–500. [Google Scholar] [CrossRef]
- Sun, J.; Wu, D.; Liu, Y.; Dai, L.; Jiang, C. Numerical simulation and experimental study of filling process of micro prism by isothermal hot embossing in solid-like state. Adv. Polym. Tech. 2018, 37, 1581–1591. [Google Scholar] [CrossRef]
- Wu, D.; Sun, J.; Liu, Y.; Yang, Z.; Xu, H.; Zheng, X.; Gou, P. Rapid fabrication of microstructure on PMMA substrate by the plate to plate Transition-Spanning isothermal hot embossing method nearby glass transition temperature. Polym. Eng. Sci. 2017, 57, 268–274. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Wan, C.; Zheng, X.; Kormakov, S.; Gao, X.; Sun, J.; Zheng, X.; Wu, D. Enhancing thermal conductivity of polydimethylsiloxane composites through spatially confined network of hybrid fillers. Compos. Sci. Technol. 2019, 172, 163–171. [Google Scholar] [CrossRef]
- Sun, J.Y.; Wang, X.B.; Wu, J.H.; Jiang, C.; Shen, J.J.; Cooper, M.A.; Zheng, X.T.; Liu, Y.; Yang, Z.G.; Wu, D.M. Biomimetic Moth-eye Nanofabrication: Enhanced Antireflection with Superior Self-cleaning Characteristic. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Wu, H.W.; Zhu, J.; Huang, Y.; Wu, D.M.; Sun, J.Y. Microfluidic-Based Single-Cell Study: Current Status and Future Perspective. Molecules 2018, 23, 2347. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Bae, J.; Hassan, A.; Ali, S.; Lee, C.H.; Choi, Y. Ink-jet printed stretchable strain sensor based on graphene/ZnO composite on micro-random ridged PDMS substrate. Compos. Part A Appl. Sci. Manuf. 2018, 107, 519–528. [Google Scholar] [CrossRef]
- Wang, D.; Ba, D.; Hao, Z.; Li, Y.; Sun, F.; Liu, K.; Du, G.; Mei, Q. A novel approach for PDMS thin films production towards application as substrate for flexible biosensors. Mater. Lett. 2018, 221, 228–231. [Google Scholar] [CrossRef]
- Wu, D.; Gao, X.; Sun, J.; Wu, D.; Liu, Y.; Kormakov, S.; Zheng, X.; Wu, L.; Huang, Y.; Guo, Z. Spatial Confining Forced Network Assembly for preparation of high-performance conductive polymeric composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 88–95. [Google Scholar] [CrossRef]
- Cui, J.L.; Zhang, B.Z.; Duan, J.P.; Guo, H.; Tang, J. Flexible Pressure Sensor with Ag Wrinkled Electrodes Based on PDMS Substrate. Sensors 2016, 16, 2131. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.H.; Kim, H.J.; Lee, S.M.; Kim, T.W.; Kim, H.K. Stretchable Ag electrodes with mechanically tunable optical transmittance on wavy-patterned PDMS substrates. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Jeong, H.; Baek, S.; Han, S.; Jang, H.; Kim, S.H.; Lee, H.S. Novel Eco-Friendly Starch Paper for Use in Flexible, Transparent, and Disposable Organic Electronics. Adv. Funct. Mater. 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Misman, M.A.; Azura, A.R.; Sidek, O. Validation of an Electronic Sensor Network (ESN) Control Chamber for Monitoring the Soil Decomposition Process of Sago Starch-filled Natural Rubber Latex Films. J. Test. Eval. 2015, 43, 1–10. [Google Scholar] [CrossRef]
- Lin, Y.H.; Kang, P.L.; Xin, W.; Yen, C.S.; Hwang, L.C.; Chen, C.J.; Liu, J.T.; Chang, S.J. Preparation and evaluation of chitosan biocompatible electronic skin. Comput. Ind. 2018, 100, 1–6. [Google Scholar] [CrossRef]
- Chao, J.Y.; Zhu, L.Q.; Xiao, H.; Yuan, Z.G. Protonic/electronic hybrid oxide transistor gated by chitosan and its full-swing low voltage inverter applications. J. Appl. Phys. 2015, 118, 1–5. [Google Scholar] [CrossRef]
- Chang, J.-W.; Wang, C.-G.; Huang, C.-Y.; Tsai, T.-D.; Guo, T.-F.; Wen, T.-C. Chicken Albumen Dielectrics in Organic Field-Effect Transistors. Adv. Mater. 2011, 23, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Najafabadi, A.H.; Tamayol, A.; Annabi, N.; Ochoa, M.; Mostafalu, P.; Akbari, M.; Nikkhah, M.; Rahimi, R.; Dokmeci, M.R.; Sonkusale, S.; et al. Biodegradable Nanofibrous Polymeric Substrates for Generating Elastic and Flexible Electronics. Adv. Mater. 2014, 26, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Xu, H.; Huang, Y.; Sun, J.Y.; Wu, D.M.; Gao, X.L.; Zhang, Y.J. Measuring Mechanism and Applications of Polymer-Based Flexible Sensors. Sensors 2019, 19, 1403. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W.; Joly, G.D.; Swager, T.M.J.C.R. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Deng, Y.; Roudsari, A.F.; Kapetanovic, A.; Anantram, M.; Rolandi, M. A polysaccharide bioprotonic field-effect transistor. Nat. Commun. 2011, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [Green Version]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; D’Amico, F.; Morresi, L.; Pinto, N.; Ficcadenti, M.; Natali, R.; Ottaviano, L.; Passacantando, M.; Cuccioloni, M.; Angeletti, M.; et al. Structural, electrical, electronic and optical properties of melanin films. Eur. Phys. J. E 2009, 28, 285–291. [Google Scholar] [CrossRef]
- Vahidzadeh, E.; Kalra, A.P.; Shankar, K. Melanin-based electronics: From proton conductors to photovoltaics and beyond. Biosens. Bioelectron. 2018, 122, 127–139. [Google Scholar] [CrossRef]
- Sheliakina, M.; Mostert, A.B.; Meredith, P. Decoupling Ionic and Electronic Currents in Melanin. Adv. Funct. Mater. 2018, 28, 1–7. [Google Scholar] [CrossRef]
- Han, J.Q.; Lu, K.Y.; Yue, Y.Y.; Mei, C.T.; Huang, C.B.; Wu, Q.L.; Xu, X.W. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crop. Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Hashemi, M.; Rahmanifar, M.S.; El-Kady, M.F.; Noori, A.; Mousavi, M.F.; Kaner, R.B. The use of an electrocatalytic redox electrolyte for pushing the energy density boundary of a flexible polyaniline electrode to a new limit. Nano Energy 2018, 44, 489–498. [Google Scholar] [CrossRef]
- Liu, T.Y.; Finn, L.; Yu, M.H.; Wang, H.Y.; Zhai, T.; Lu, X.H.; Tong, Y.X.; Li, Y. Polyaniline and Polypyrrole Pseudocapacitor Electrodes with Excellent Cycling Stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Karthik, K.V.; Prasad, S.B.B.; Soni, S.K.; Jeong, H.M.; Raghu, A.V. Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Huang, G.W.; Xiao, H.M.; Fu, S.Y. Electrical Switch for Smart pH Self-Adjusting System Based on Silver Nanowire/Polyaniline Nanocomposite Film. ACS Nano 2015, 9, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Kamalesh, S.; Tan, P.; Wang, J.; Lee, T.; Kang, E.-T.; Wang, C.-H. Biocompatibility of electroactive polymers in tissues. J. Biomed. Mater. Res. 2000, 52, 467–478. [Google Scholar] [CrossRef]
- Song, H.-K.; Toste, B.; Ahmann, K.; Hoffman-Kim, D.; Palmore, G. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: A new platform for characterizing neurite extension in complex environments. Biomaterials 2006, 27, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.L.; Yuan, X.C.; Li, Z.J.; Zhao, K.; Sheng, L.Y.; Li, Q.D. Direct growth of 3D porous (Ni-Co)(3)S(4 )nanosheets arrays on rGO-PEDOT hybrid film for high performance non-enzymatic glucose sensing. Sensor Actuat. B-Chem. 2019, 291, 9–16. [Google Scholar] [CrossRef]
- Nitta, K.; Tsumaki, M.; Kawano, T.; Terashima, K.; Ito, T. Printing PEDOT from EDOT via plasma-assisted inkjet printing. J. Phys. D. Appl. Phys. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Abidian, M.R.; Ludwig, K.A.; Marzullo, T.C.; Martin, D.C.; Kipke, D.R. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly (3,4-ethylenedioxythiophene) Nanotubes. Adv. Mater. 2009, 21, 3764–3770. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Burns, S.M.; Hendricks, J.L.; Foster, B.; Povlich, L.K.; Kim, D.-H.; Martin, D.C. Polymerization of the conducting polymer poly (3, 4-ethylenedioxythiophene)(PEDOT) around living neural cells. Biomaterials. 2007, 28, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Mahato, S.; Gerling, L.G.; Voz, C.; Alcubilla, R.; Puigdollers, J. High efficiency ITO-free hybrid solar cell using highly conductive PEDOT:PSS with co-solvent and surfactant treatments. Mater. Lett. 2017, 186, 165–167. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Herve, T.; Sanaur, S.; Bernard, C.; Malliaras, G.G. Highly Conformable Conducting Polymer Electrodes for In Vivo Recordings. Adv. Mater. 2011, 23, H268–H272. [Google Scholar] [CrossRef]
- Yang, H.; Bai, S.C.; Chen, T.R.; Zhang, Y.; Wang, H.F.; Guo, X.Z. Facile fabrication of large-scale silver nanowire-PEDOT:PSS composite flexible transparent electrodes for flexible touch panels. Mater. Res. Express 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Duran, N.; Paula Lemes, A.; B Seabra, A. Review of cellulose nanocrystals patents: preparation, composites and general applications. Recent Pat. Nanotech. 2012, 6, 16–28. [Google Scholar] [CrossRef]
- Zhu, H.; Fang, Z.; Preston, C.; Li, Y.; Hu, L. Transparent paper: fabrications, properties, and device applications. Energy Environ. Sci. 2014, 7, 269–287. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose-Its barrier properties and applications in cellulosic materials: A review. Carbohyd. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohyd. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohyd. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.; Capadona, J.; Rowan, S.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Svagan, A.J.; Busko, D.; Avlasevich, Y.; Glasser, G.; Baluschev, S.; Landfester, K. Photon energy upconverting nanopaper: a bioinspired oxygen protection strategy. ACS Nano 2014, 8, 8198–8207. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, P.; Michen, B.; Huch, A.; Tingaut, P.; Caseri, W.R.; Zimmermann, T. Characterization of pores in dense nanopapers and nanofibrillated cellulose membranes: a critical assessment of established methods. ACS Appl. Mater. Interfaces 2015, 7, 25884–25897. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhu, H.; Zhong, Q.; Dai, J.; Li, W.; Jang, S.-H.; Yao, Y.; Henderson, D.; Hu, Q.; Hu, L. Self-powered human-interactive transparent nanopaper systems. ACS Nano 2015, 9, 7399–7406. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Mori, T.; Tsuruta, R.; Yamanaka, S.; Yoshida, K.; Imai, K.; Koganezawa, T.; Hosokai, T. Surface crystallographic structures of cellulose nanofiber films and overlayers of pentacene. Jpn. J. Appl. Phys. 2018, 57, 1–4. [Google Scholar] [CrossRef]
- Park, J.; Seo, J.-H.; Yeom, S.-W.; Yao, C.; Yang, V.W.; Cai, Z.; Jhon, Y.M.; Ju, B.-K. Flexible and Transparent Organic Phototransistors on Biodegradable Cellulose Nanofibrillated Fiber Substrates. Adv. Opt. Mater. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Ye, D.D.; Yang, W.T.; Zhang, S.H.; Chen, H.Z.; Chang, C.Y.; Zhang, L.N. Construction of Transparent Cellulose-Based Nanocomposite Papers and Potential Application in Flexible Solar Cells. ACS Sustain. Chem. Eng. 2018, 6, 8040–8047. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.H.; Zhang, H.L.; Yao, C.H.; Zheng, Q.F.; Yang, V.W.; Mi, H.Y.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Kormakov, S.; He, X.; Gao, X.; Zheng, X.; Liu, Y.; Sun, J.; Wu, D. Conductive Polymer Composites from Renewable Resources: An Overview of Preparation, Properties, and Applications. Polymers 2019, 11, 187. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Liu, Y.; Zheng, X.; Kormakov, S.; Sun, J.; Zhuang, J.; Gao, X.; Wu, D. Improved thermal conductivity of polydimethylsiloxane/short carbon fiber composites prepared by spatial confining forced network assembly. J. Mater. Sci. 2018, 53, 14299–14310. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, J.; Shi, J.; Kormakov, S.; Liu, Y.; Yang, Z.; Wu, D. Highly elastic and ultrathin nanopaper-based nanocomposites with superior electric and thermal characteristics. J. Mater. Sci. 2019, 54, 8436–8449. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Yang, Z.; Shen, J.; Cabrera, E.; Lertola, M.J.; Yang, W.; Zhang, D.; Benatar, A.; Castro, J.M.; et al. Highly stretchable and ultrathin nanopaper composites for epidermal strain sensors. Nanotechnology 2018, 29, 355304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cabrera, E.D.; Zhang, D.; Sun, J.; Kuang, T.; Yang, W.; Lertola, M.J.; Benatar, A.; Castro, J.M.; Lee, L.J. Ultrasonic processing of MWCNT nanopaper reinforced polymeric nanocomposites. Polymer 2018, 156, 85–94. [Google Scholar] [CrossRef]
- Ramadas, M.; Bharath, G.; Ponpandian, N.; Ballamurugan, A. Investigation on biophysical properties of Hydroxyapatite/Graphene oxide (HAp/GO) based binary nanocomposite for biomedical applications. Mater. Chem. Phys. 2017, 199, 179–184. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L.J.J.O.M.S. A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: a review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Sherlala, A.; Raman, A.; Bello, M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Ling, S.; Wang, Q.; Zhang, D.; Zhang, Y.; Mu, X.; Kaplan, D.L.; Buehler, M.J. Integration of stiff graphene and tough silk for the design and fabrication of versatile electronic materials. Adv. Funct. Mater. 2018, 28, 1705291. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lo Re, G.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The Advances of Carbon Nanotubes in Cancer Diagnostics and Therapeutics. J. Nanomater. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Daenen, M.; Zhang, L.; Erni, R.; Williams, O.A.; Hardy, A.; Van Bael, M.K.; Wagner, P.; Haenen, K.; Nesládek, M.; Van Tendeloo, G. Diamond Nucleation by Carbon Transport from Buried Nanodiamond TiO2 Sol-Gel Composites. Adv. Mater. 2009, 21, 670–673. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T. Multiwalled carbon nanotubes and sepiolite nanoclays as flame retardants for polylactide and its natural fibre reinforced composites. Compos. Part A: Appl. Sci. Manuf. 2010, 41, 954–963. [Google Scholar] [CrossRef]
- Ma, P.; Jiang, L.; Ye, T.; Dong, W.; Chen, M. Melt free-radical grafting of maleic anhydride onto biodegradable poly (lactic acid) by using styrene as a comonomer. Polymers 2014, 6, 1528–1543. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, H.S.; Kim, J.H.; Shin, U.S.; Lee, S.H. Carbon Nanotube Nanocomposites with Highly Enhanced Strength and Conductivity for Flexible Electric Circuits. Langmuir 2015, 31, 7844–7851. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, N.R.; Peshkov, D.A.; D’Yachkov, P.N. Combined Effect of External Periodic and Constant Electric Fields on Electron Transport in Carbon Nanotubes and Nanoribbons with Metallic Conductivity. J. Phys. Soc. Jpn. 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Badard, M.; Combessis, A.; Allais, A.; Flandin, L. Electric field as a tuning key to process carbon nanotube suspensions with controlled conductivity. Polymer 2016, 82, 198–205. [Google Scholar] [CrossRef]
- Osazuwa, O.; Vasileiou, A.A.; Kontopoulou, M.; Docoslis, A. Electric-field induced filler association dynamics and resulting improvements in the electrical conductivity of polyester/multiwall carbon nanotube composites. Polym. Compos. 2017, 38, 1571–1578. [Google Scholar] [CrossRef]
- Valentini, L.; Fabbri, P.; Messori, M.; Degli Esposti, M.; Bon, S.B. Multilayer Films Composed of Conductive Poly(3-hydroxybutyrate)/Carbon Nanotubes Bionanocomposites and a Photoresponsive Conducting Polymer. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 596–602. [Google Scholar] [CrossRef]
- Dionigi, C.; Posati, T.; Benfenati, V.; Sagnella, A.; Pistone, A.; Bonetti, S.; Ruani, G.; Dinelli, F.; Padeletti, G.; Zamboni, R.; et al. A nanostructured conductive bio-composite of silk fibroin-single walled carbon nanotubes. J. Mater. Chem. B 2014, 2, 1424–1431. [Google Scholar] [CrossRef]
- Sivanjineyulu, V.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Selective localization of carbon nanotube and organoclay in biodegradable poly(butylene succinate)/polylactide blend-based nanocomposites with enhanced rigidity, toughness and electrical conductivity. Compos. Part A Appl. Sci. Manuf. 2018, 114, 30–39. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Tan, Y.-J.; Li, J.; Hao, Y.-B.; Shi, Y.-D.; Wang, M. Graphene oxide-assisted dispersion of multi-walled carbon nanotubes in biodegradable Poly(epsilon-caprolactone) for mechanical and electrically conductive enhancement. Polym. Test. 2018, 65, 387–397. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Ge, J.; Xue, Y.; Niu, W.; Chen, M.; Du, Y.; Ma, P.X.; Lei, B. Biodegradable thermal imaging-tracked ultralong nanowire-reinforced conductive nanocomposites elastomers with intrinsical efficient antibacterial and anticancer activity for enhanced biomedical application potential. Biomaterials 2019, 201, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent Progress in Metal-Based Nanoparticles Mediated Photodynamic Therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Behera, D.; Rath, P.; Bastia, T.K. Enhanced properties of UPE/ESOA partially bio-nanocomposites reinforced with chitosan functionalized graphene nanoplatelets: An innovative approach. Bull. Mater. Sci. 2018, 41, 12. [Google Scholar] [CrossRef]
- Sim, S.; Andou, Y.; Bashid, H.A.A.; Lim, H.; Altarawneh, M.; Jiang, Z.T.; Eksiler, K.; Iikubo, S. Development of Organo-Dispersible Graphene Oxide via Pseudo-Surface Modification for Thermally Conductive Green Polymer Composites. ACS Omega 2018, 3, 18124–18131. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, D.G.; Boyer, I.; Devahif, T.; Gao, C.; Frank, B.P.; Lu, X.; Kuwama, L.; Gordon, T.B.; Wang, J.L.; Ranville, J.F.; et al. Biodegradation of Carbon Nanotube/Polymer Nanocomposites using a Monoculture. Environ. Sci. Technol. 2018, 52, 40–51. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhu, L.T.; Wang, Z.B.; Zong, L.; Li, M.J.; Wu, X.C.; Li, C.X. Liquid exfoliated chitin nanofibrils for re-dispersibility and hybridization of two-dimensional nanomaterials. Chem. Eng. J. 2018, 344, 498–505. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Mohseni, M. Biodegradable polypyrrole/dextrin conductive nanocomposite: Synthesis, characterization, antioxidant and antibacterial activity. Synth. Met. 2014, 187, 9–16. [Google Scholar] [CrossRef]
- Du, Y.Z.; Ge, J.; Li, Y.N.; Ma, P.X.; Lei, B. Biomimetic elastomeric, conductive and biodegradable polycitrate-based nanocomposites for guiding myogenic differentiation and skeletal muscle regeneration. Biomaterials 2018, 157, 40–50. [Google Scholar] [CrossRef]
- Mohan, R.; Subha, J.; Alam, J. Influence of Multiwalled Carbon Nanotubes on Biodegradable Poly(lactic acid) Nanocomposites for Electroactive Shape Memory Actuator. Adv. Polym. Tech. 2018, 37, 1–6. [Google Scholar] [CrossRef]
- Lao, J.P.; Xie, H.A.; Shi, Z.Q.; Li, G.; Li, B.; Hu, G.H.; Yang, Q.L.; Xiong, C.X. Flexible Regenerated Cellulose/Boron Nitride Nanosheet High-Temperature Dielectric Nanocomposite Films with High Energy Density and Breakdown Strength. ACS Sustain. Chem. Eng. 2018, 6, 7151–7158. [Google Scholar] [CrossRef]
- Pawde, S.; Deshmukh, K. Influence of γ irradiation on the properties of polyacrylonitrile films. J. Appl. Polym. Sci. 2008, 110, 2569–2578. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Pasha, S.K.; Deshmukh, R.R.; Bhagat, P.R. Highly dispersible graphene oxide reinforced polypyrrole/polyvinyl alcohol blend nanocomposites with high dielectric constant and low dielectric loss. RSC Adv. 2015, 5, 61933–61945. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Dielectric properties and electromagnetic interference shielding effectiveness of graphene-based biodegradable nanocomposites. Mater. Design. 2016, 109, 68–78. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.K.; Sadasivuni, K.K.; Polu, A.R.; Ponnamma, D.; AlMaadeed, M.A.-A.; Chidambaram, K. Newly developed biodegradable polymer nanocomposites of cellulose acetate and Al2O3 nanoparticles with enhanced dielectric performance for embedded passive applications. J. Mater. Sci.-Mater. Electron. 2017, 28, 973–986. [Google Scholar] [CrossRef]
- Zeng, X.; Deng, L.; Yao, Y.; Sun, R.; Xu, J.; Wong, C.-P. Flexible dielectric papers based on biodegradable cellulose nanofibers and carbon nanotubes for dielectric energy storage. J. Mater. Chem. C 2016, 4, 6037–6044. [Google Scholar] [CrossRef]
- Choudhary, S. Characterization of amorphous silica nanofiller effect on the structural, morphological, optical, thermal, dielectric and electrical properties of PVA-PVP blend based polymer nanocomposites for their flexible nanodielectric applications. J. Mater. Sci.-Mater. Electron. 2018, 29, 10517–10534. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Sadasivuni, K.K.; Ponnamma, D.; AlMaadeed, M.A.A.; Deshmukh, R.R.; Pasha, S.K.K.; Polu, A.R.; Chidambaram, K. Fumed SiO2 nanoparticle reinforced biopolymer blend nanocomposites with high dielectric constant and low dielectric loss for flexible organic electronics. J. Appl. Polym. Sci. 2017, 134, 1–11. [Google Scholar] [CrossRef]
- Feili, D.; Schuettler, M.; Doerge, T.; Kammer, S.; Stieglitz, T. Encapsulation of organic field effect transistors for flexible biomedical microimplants. Sens. Actuators A Phys. 2005, 120, 101–109. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, J.; Kim, Y.; Kwak, J.W.; Yoon, Y.; Rogers, J.A. Room temperature electrochemical sintering of Zn microparticles and its use in printable conducting inks for bioresorbable electronics. Adv. Mater. 2017, 29, 1702665. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Y.; Teng, J.; Wang, J.; Nee, T. Preparation and characterization of pentacene-based organic thin-film transistors with PVA passivation layers. Thin Solid Films 2009, 517, 5318–5321. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Z.; Zhou, C.; Zhu, J.; Lee, R.J.; Teng, L. Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 2016, 34, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Huang, Y.; Zheng, X.; Liu, Y.; Zhuang, J.; Wu, D. Simple and Affordable Way To Achieve Polymeric Superhydrophobic Surfaces with Biomimetic Hierarchical Roughness. ACS Omega 2019, 4, 2750–2757. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, K.; Wang, Z.; Zhao, Q.; Yang, Y.; Zhang, Y.; Ai, S.; Xu, J. Biodegradable poly(vinyl alcohol)-based nanocomposite film reinforced with organophilic layered double hydroxides with potential packaging application. Iran. Polym. J. 2017, 26, 811–819. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Zhao, Q.; Yang, Y.; Xu, J.; Waterhouse, G.I.N.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-Up Fabrication of Biodegradable Poly(butylene adipate-co-terephthalate)/Organophilic-Clay Nanocomposite Films for Potential Packaging Applications. ACS Omega 2018, 3, 1187–1196. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, K.; Wu, J.; Ren, G.; Chen, H.; Xu, J. Bio-nanocomposite films reinforced with organo-modified layered double hydroxides: Preparation, morphology and properties. Appl. Clay. Sci. 2016, 126, 72–80. [Google Scholar] [CrossRef]
- Wang, S.; Jing, Y. Effects of formation and penetration properties of biodegradable montmorillonite/chitosan nanocomposite film on the barrier of package paper. Appl. Clay. Sci. 2017, 138, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.G.; Liu, X.H.; Ren, F.; Zhong, G.J.; Ji, X.; Xu, L. Biodegradable graphene oxide nanosheets/poly-(butylene adipate-coterephthalate) nanocomposite film with enhanced gas and water vapor barrier properties. Polym. Test. 2017, 58, 173–180. [Google Scholar] [CrossRef]
- Pawar, S.P.; Kumar, S.; Misra, A.; Deshmukh, S.; Chatterjee, K.; Bose, S. Enzymatically degradable EMI shielding materials derived from PCL based nanocomposites. RSC Adv. 2015, 5, 17716–17725. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.K.; Singh, B.P.; Mathur, R.B.; Dhakate, S.R. Multi-walled carbon nanotube–graphene–polyaniline multiphase nanocomposite with superior electromagnetic shielding effectiveness. Nanoscale 2014, 6, 842–851. [Google Scholar] [CrossRef]
- Kuang, T.R.; Chang, L.Q.; Chen, F.; Sheng, Y.; Fu, D.J.; Peng, X.F. Facile preparation of lightweight high-strength biodegradable polymer/multi-walled carbon nanotubes nanocomposite foams for electromagnetic interference shielding. Carbon 2016, 105, 305–313. [Google Scholar] [CrossRef]
- Wang, G.L.; Zhao, G.Q.; Wang, S.; Zhang, L.; Park, C.B. Injection-molded microcellular PLA/graphite nanocomposites with dramatically enhanced mechanical and electrical properties for ultra-efficient EMI shielding applications. J. Mater. Chem. C 2018, 6, 6847–6859. [Google Scholar] [CrossRef]
- Shih, Y.F.; Chen, L.S.; Jeng, R.J. Preparation and properties of biodegradable PBS/multi-walled carbon nanotube nanocomposites. Polymer 2008, 49, 4602–4611. [Google Scholar] [CrossRef]





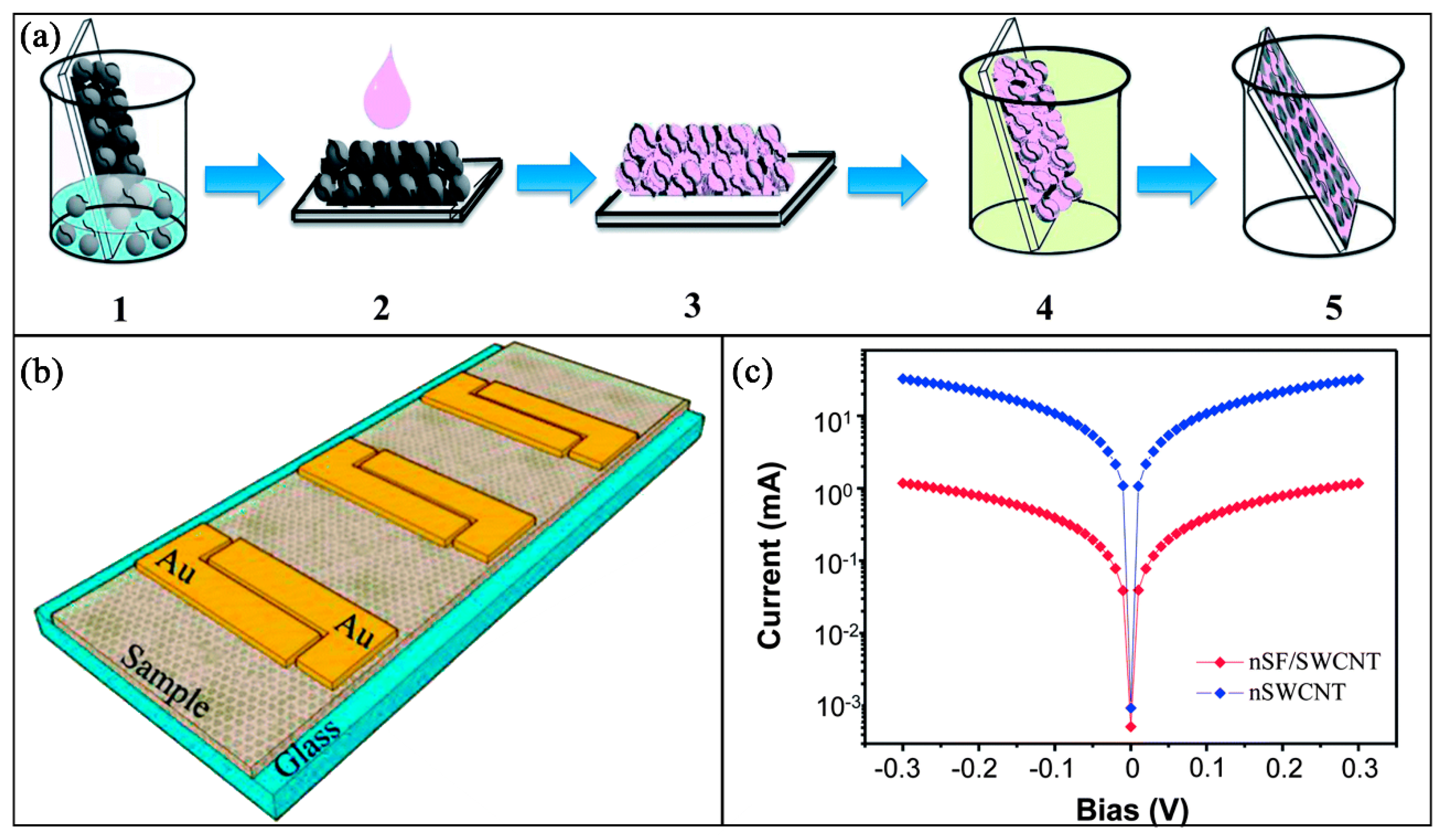
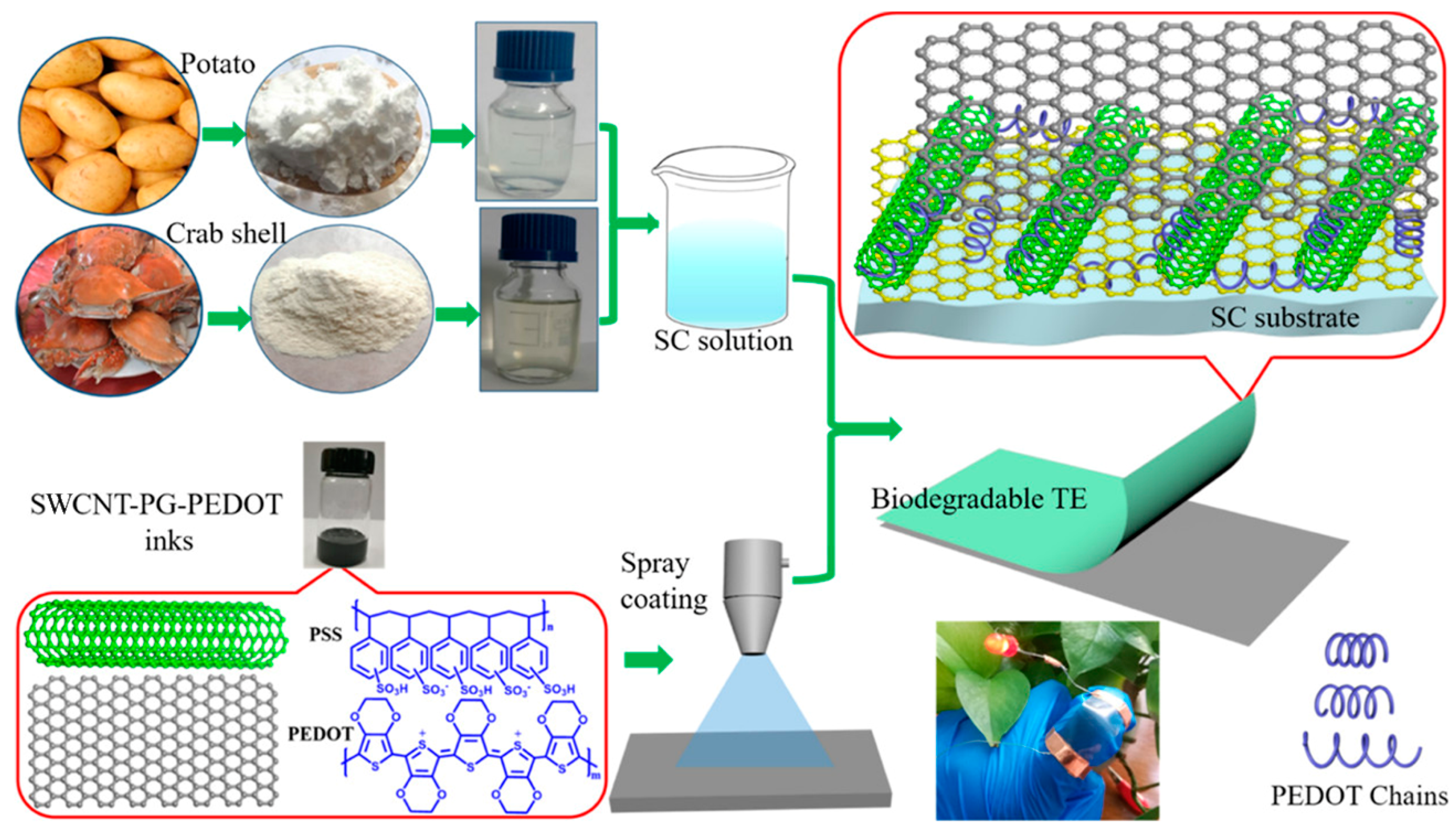







| Category | Polymer Material | Electrical Property | Biodegradable/Biocompatible | Applications |
|---|---|---|---|---|
| Natural Polymers | Cellulose | Insulator | Both | Substrate [24,25]; Dielectric [26] |
| Silk | Insulator | Both | Substrate [27,28]; Dielectric [29] | |
| Shellac | Insulator | Both | Substrate [30]; Dielectric [30,31] | |
| Gelatin | Insulator | Both | Substrate [32,33]; Dielectric [34,35,36] | |
| Synthetic Polymer | Poly(vinyl alcohol) (PVA) | Insulator | Biocompatible | Substrate [37,38]; Dielectric [39,40] |
| Polydimethylsiloxane (PDMS) | Insulator | Biocompatible | Substrate [41]; Dielectric [42,43,44,45] | |
| Polylactide (PLA) | Insulator | Both | Substrate [46,47,48]; Dielectric [49] | |
| Polycaprolactone (PCL) | Insulator | Both | Dielectric [49] | |
| Poly(glycerol-co-sebacate) (PGS) | Insulator | Both | Dielectric [50] | |
| Poly(lactic-co-glycolic acid) (PLGA) | Insulator | Both | Substrate [51]; Dielectric [52] | |
| Polyaniline (PANI) | Conductor (doped) | Biocompatible | Conductor [53] | |
| Polypyrrole (PPy) | Conductor (doped) | Biocompatible | Conductor [54] | |
| Poly(3,4-ethylenedioxythiophene) (PEDOT) | Conductor (doped) | Biocompatible | Conductor [55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Jian, R.; Chen, H.; Tian, X.; Sun, C.; Zhu, J.; Yang, Z.; Sun, J.; Wang, C. Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions. Nanomaterials 2019, 9, 950. https://doi.org/10.3390/nano9070950
Liu H, Jian R, Chen H, Tian X, Sun C, Zhu J, Yang Z, Sun J, Wang C. Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions. Nanomaterials. 2019; 9(7):950. https://doi.org/10.3390/nano9070950
Chicago/Turabian StyleLiu, Haichao, Ranran Jian, Hongbo Chen, Xiaolong Tian, Changlong Sun, Jing Zhu, Zhaogang Yang, Jingyao Sun, and Chuansheng Wang. 2019. "Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions" Nanomaterials 9, no. 7: 950. https://doi.org/10.3390/nano9070950
APA StyleLiu, H., Jian, R., Chen, H., Tian, X., Sun, C., Zhu, J., Yang, Z., Sun, J., & Wang, C. (2019). Application of Biodegradable and Biocompatible Nanocomposites in Electronics: Current Status and Future Directions. Nanomaterials, 9(7), 950. https://doi.org/10.3390/nano9070950






