Preparation of Micro-Nano Material Composed of Oyster Shell/Fe3O4 Nanoparticles/Humic Acid and Its Application in Selective Removal of Hg(II)
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Oyster Shell (OS)
2.2. Synthesis of Magnetic Nanomaterials
2.3. Adsorption Experiments
2.4. Experimental Analyses
3. Results and Discussion
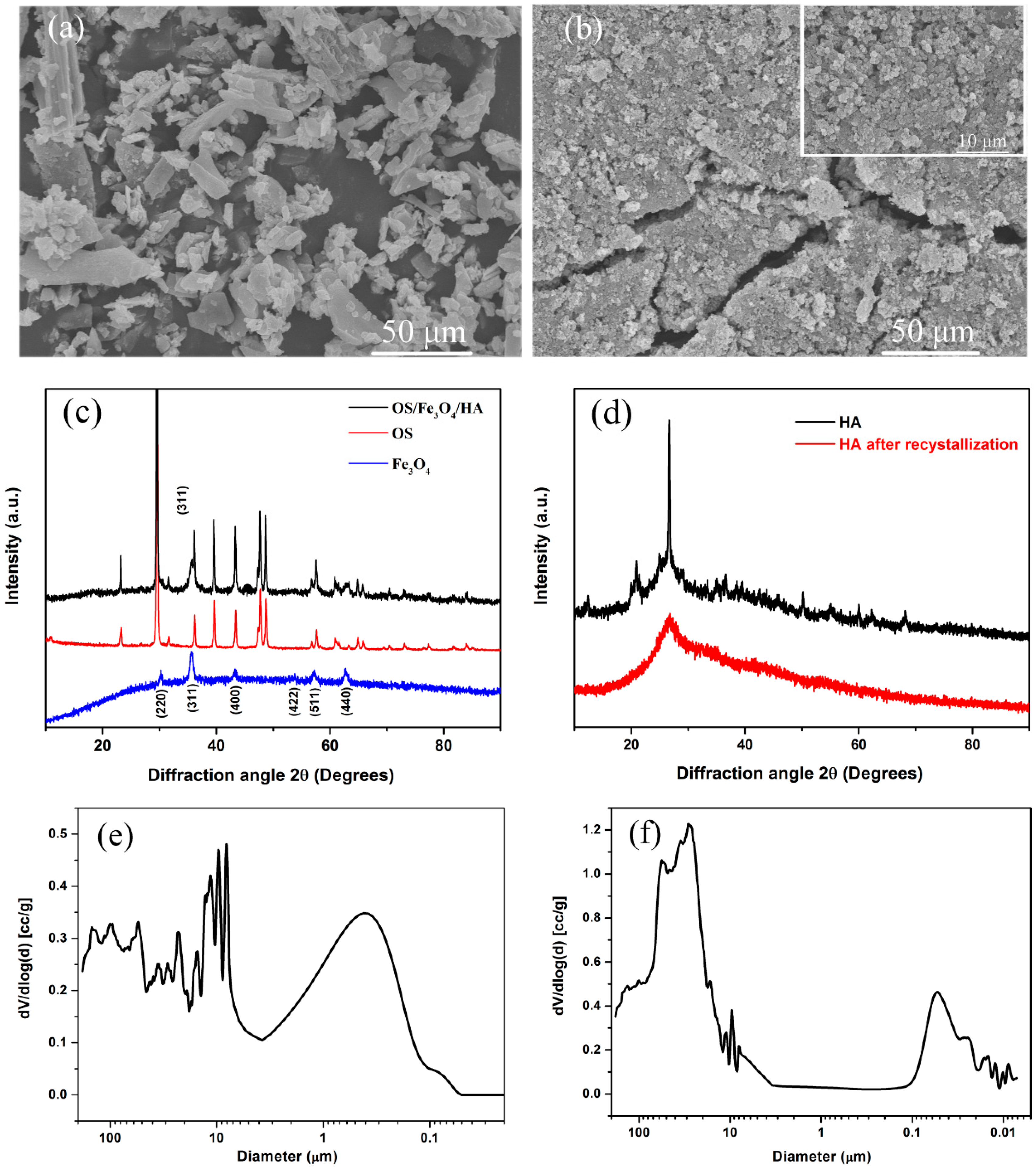
3.1. Material Characterization
3.2. Optimization of pH in Hg(II) Ion Adsorption
3.3. Adsorption Isotherms
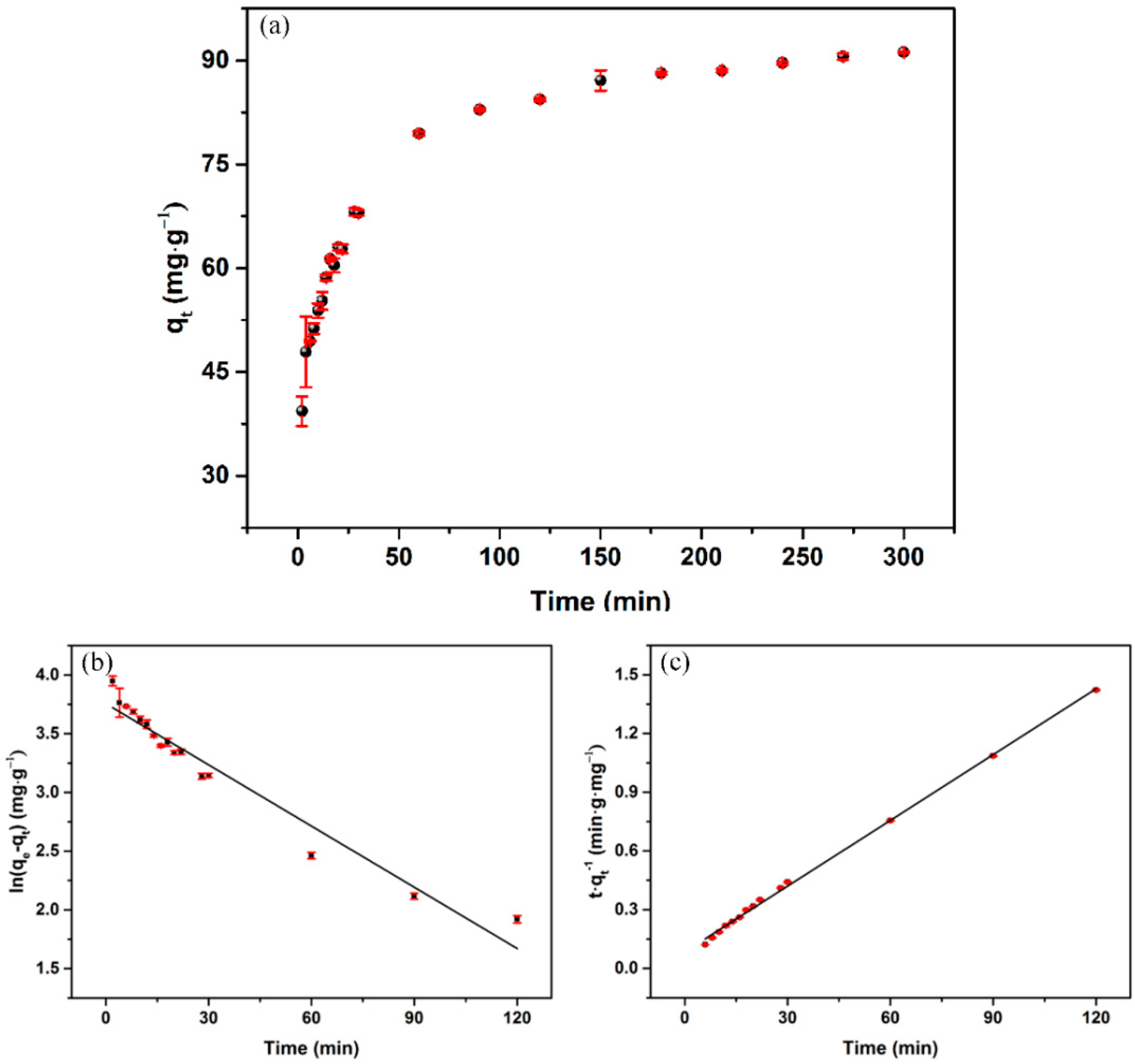
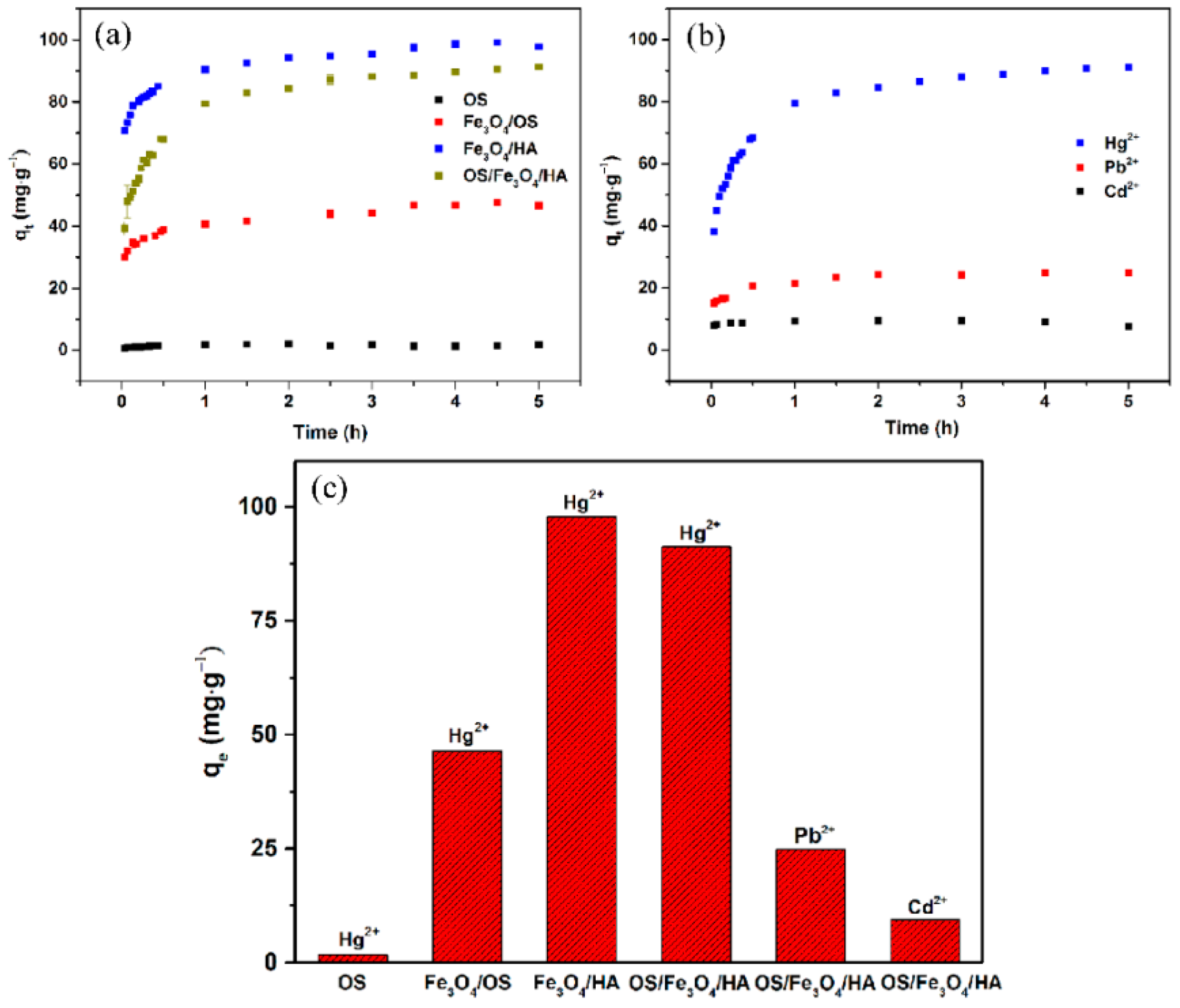
3.4. Adsorption Kinetics
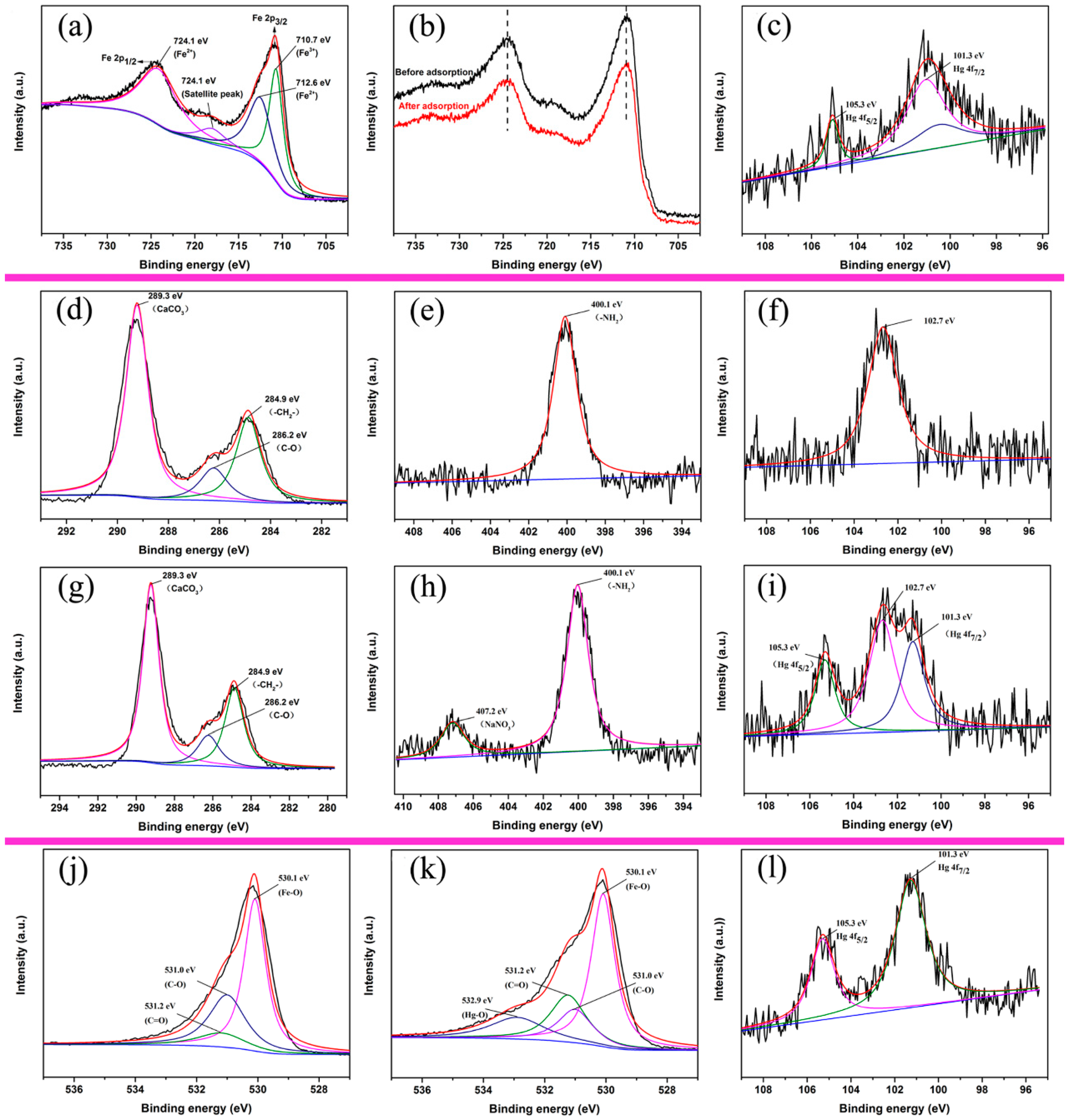
3.5. Adsorption Mechanism
3.6. Efficient and Selective Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mamdani, H.; Vettese, T.E. Images in clinical medicine. Pulmonary emboli caused by mercury. N. Engl. J. Med. 2013, 369, 2031. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; James, K.A.; Levy, L.S. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Rissanen, T.H.; Voutilainen, S.; Tuomainen, T.P. Mercury as a risk factor for cardiovascular diseases. J. Nutr. Biochem. 2007, 18, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hou, L.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Wu, H.; Chen, M.; Peng, H.; Li, B.; Liu, R.; Liu, Z.; Wang, B.; Huang, T.; Hu, Z. Fractional removal of manganese and ammonia nitrogen from electrolytic metal manganese residue leachate using carbonate and struvite precipitation. Water Res. 2018, 153, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Song, X.; Fang, X.; Yang, W.; Hao, X.; Iritani, E.; Katagiri, N. Membrane filtration-based recovery of extracellular polymer substances from excess sludge and analysis of their heavy metal ion adsorption properties. Chem. Eng. J. 2018, 354, 866–874. [Google Scholar] [CrossRef]
- Hong, G.; Shen, L.; Wang, M.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Nanofibrous polydopamine complex membranes for adsorption of Lanthanum (III) ions. Chem. Eng. J. 2014, 244, 307–316. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Impacts of coagulation-flocculation treatment on the size distribution and bioavailability of trace metals (Cu, Pb, Ni, Zn) in municipal wastewater. Water Res. 2018, 128, 120–128. [Google Scholar]
- Deliyanni, E.A.; Kyzas, G.Z.; Matis, K.A. Various flotation techniques for metal ions removal. J. Mol. Liq. 2017, 225, 260–264. [Google Scholar] [CrossRef]
- Tran, T.; Chiu, K.; Lin, C.; Leu, H. Electrochemical treatment of wastewater: Selectivity of the heavy metals removal process. Int. J. Hydrogen Energy 2017, 42, 27741–27748. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Li, R.; Dai, Q.; Gao, R.; Liu, Q.; Zhang, M. Preparation of Fe3O4@C@layered double hydroxide composite for magnetic separation ofuranium. Ind. Eng. Chem. Res. 2013, 52, 10152–10159. [Google Scholar] [CrossRef]
- Tan, L.; Wang, J.; Liu, Q.; Sun, Y.; Zhang, H.; Wang, Y.; Jing, X.; Liu, J.; Song, D. Facile preparation of oxine functionalized magnetic Fe3O4 particles for enhanced uranium (VI) adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 85–91. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Jiang, G. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhang, D.; Zhang, S.; Zhang, X.; Meng, Z.; Cai, Y. Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J. Hazard. Mater. 2011, 190, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, A.W.P.; Riemsdijk, W.H.V.; Koopal, L.K. Adsorption of humic acid to mineral particles. 1. specific and electrostatic interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.; Clark, M.; Yu, Y. Adsorption of copper to different biogenic oyster shell structures. Appl. Surf. Sci. 2014, 311, 264–272. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, I.; Bajpai, V.K.; Lee, H.; Kim, T.; Upadhyay, A.; Huh, Y.S.; Han, Y.; Tripathi, K.M. Sustainable graphene aerogel as an ecofriendly cell growth promoter and highly efficient adsorbent for histamine from red wine. ACS Appl. Mater. Interfaces 2019, 11, 18165–18177. [Google Scholar] [CrossRef]
- Myung, Y.; Jung, S.; Tung, T.T.; Tripathi, K.M.; Kim, T. Graphene-based aerogels derived from biomass for energy storage and environmental remediation. ACS Sustain. Chem. Eng. 2019, 7, 3772–3782. [Google Scholar] [CrossRef]
- Hsu, T.C. Experimental assessment of adsorption of Cu2+ and Ni2+ from aqueous solution by oyster shell powder. J. Hazard. Mater. 2009, 171, 995–1000. [Google Scholar] [CrossRef]
- Li, C.; Qian, Z.; Zhou, C.; Su, W.; Hong, P.; Liu, S.; He, L.; Chen, Z.; Ji, H. Mussel-inspired synthesis of polydopamine-functionalized calcium carbonate as reusable adsorbents for heavy metal ions. RSC Adv. 2014, 4, 47848–47852. [Google Scholar] [CrossRef]
- Jung, S.; Heo, N.S.; Kim, E.J.; Oh, S.Y.; Lee, H.U.; Kim, I.T.; Hur, J.; Lee, G.; Lee, Y.; Huh, Y.S. Feasibility test of waste oyster shell powder for water treatment. Process. Saf. Environ. 2016, 102, 129–139. [Google Scholar] [CrossRef]
- Yen, H.Y.; Chou, J.H. Water purification by oyster shell bio-medium in a recirculating aquaponic system. Ecol. Eng. 2016, 95, 229–236. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, S.; Zhang, X.; Zhou, H.; Lu, Z.; Wang, S. Removal of arsenic from simulation wastewater using nano-iron/oyster shell composites. J. Environ. Manag. 2015, 156, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Jha, S.K.; Pandey, S.P.; Neogy, S. Rapid extraction of uranium from sea water using Fe3O4 and humic acid coated Fe3O4 nanoparticles. J. Hazard. Mater. 2017, 335, 152–161. [Google Scholar] [CrossRef]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [PubMed]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb (II), Hg (II) and Cu (II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, D.; Choi, S.C.; Lee, D.; Choi, J.Y.; Kim, H. Porous multi-walled carbon nanotubes by using catalytic oxidation via transition metal oxide. Microporous Mesoporous Mater. 2014, 194, 46–51. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, T.; Qiao, X.; Zhang, J.; Yang, L. Effects of synthetical conditions on octahedral magnetite nanoparticles. Mater. Sci. Eng. B 2007, 136, 101–105. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Jeong, U.; Teng, X.; Wang, Y.; Yang, H.; Xia, Y. Superparamagnetic Colloids: Controlled Synthesis and Niche Applications. Adv. Mater. 2007, 19, 33–60. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wang, L.; James, T.D.; Xiao, H.N.; Zhu, W.H. A glutamic acid-modifie cellulose fibrous composite used for the adsorption of heavy metal ions from single and binary solutions. Mater. Chem. Front. 2017, 1, 2317–2323. [Google Scholar] [CrossRef]
- Alberts, E.M.; Taylor, S.D.; Edwards, S.L.; Sherman, D.M.; Huang, C.P.; Kenny, P.; Wilker, J.J. Structural and compositional characterization of the adhesive produced by reef building oysters. ACS Appl. Mater. Interfaces 2015, 7, 8533–8538. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shapter, J.G.; Popelka-Filcoff, R.; Bennett, J.W.; Ellis, A.V. Copper removal using bio-inspired polydopamine coated natural zeolites. J. Hazard. Mater. 2014, 273, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Song, S.; Wang, X.; Niu, F.; Ma, R.; Yu, S.; Wen, T.; Chen, Y.; Hayat, T.; Alsaedi, A.; et al. Rationally designed core-shell and yolk-shell magnetic titanate nanosheets for efficient U (VI) adsorption performance. Environ. Pollut. 2018, 238, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Shan, C.; Liang, J.; Tong, M. Efficient adsorption of Selenium (IV) from water by hematite modified magnetic nanoparticles. Chemosphere 2018, 193, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, M.; Hu, Y.; Cheng, J.; Lv, S.; Yang, K. Reductive degradation of 2,2′,4,4′-tretrabromodiphenyl ether with PAC-Pd/Fe nanoparticles: Effects of Pd loading, initial pH and HA, and degradation pathway. Chem. Eng. J. 2018, 334, 1252–1259. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Fan, M.; Yang, D.; Zhu, R.; Ge, F.; Zhu, J.; He, H. Removal of hexavalent chromium [Cr (VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef]
- Terkhi, M.C.; Taleb, F.; Gossart, P.; Semmoud, A.; Addou, A. Fourier transform infrared study of mercury interaction with carboxyl groups in humic acids. J. Photochem. Photobiol. A 2008, 198, 205–214. [Google Scholar] [CrossRef]
- Hutson, N.D.; Attwood, B.C.; Scheckel, K.G. XAS and XPS characterization of mercury binding on brominated activated carbon. Environ. Sci. Technol. 2007, 41, 1747–1752. [Google Scholar] [CrossRef]
- Kushwaha, S.; Sreedhar, B.; Bhatt, R.; Sudhakar, P.P. Spectroscopic characterization for remediation of copper, cadmium and mercury using modified palm shell powder. J. Taiwan Inst. Chem. Eng. 2015, 46, 191–199. [Google Scholar] [CrossRef]
- Cui, H.; Qian, Y.; Li, Q.; Zhang, Q.; Zhai, J. Adsorption of aqueous Hg (II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012, 211, 216–223. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Yu, Y.; Qi, J.; Jia, X.; Wang, J.; Li, X. Production of unburned calcium silicon filter material (UCSFM) from oyster shell and its performance investigation in an A/O integrated biological aerated filter reactor (A/O-BAF). RSC Adv. 2016, 6, 85324–85332. [Google Scholar] [CrossRef]
- Simmons, J.M.; Nichols, B.M.; Marcus, M.S.; Castellini, O.M.; Hamers, R.J.; Eriksson, M.A. Critical oxide thickness for efficient single-walled carbon nanotube growth on silicon using thin SiO2 diffusion barriers. Small 2006, 2, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Janoš, P.; Kormunda, M.; Životský, O.; Pilařová, V. Composite Fe3O4/Humic Acid Magnetic Sorbent and its Sorption Ability for Chlorophenols and some other Aromatic Compounds. Sep. Sci. Technol. 2013, 48, 2028–2035. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Liu, J.; Xu, Q.; Xiao, H.; Wang, X.; Xu, H.; Zhou, J. Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.; Xia, K.; Zhou, X.; Guo, Y.; Huang, T. Facile synthesis of thiol-functionalized magnetic activated carbon and application for the removal of mercury (II) from aqueous solution. ACS Omega 2019, 4, 8568–8579. [Google Scholar] [CrossRef]
- Kluczka, J. Reactive Polymers in Mercury Removal from Electrolytic Brine. Sep. Sci. Technol. 2009, 44, 3698–3716. [Google Scholar] [CrossRef]
- Alijani, H.; Shariatinia, Z.; Mashhadi, A.A. Water assisted synthesis of MWCNTs over natural magnetic rock: An effective magnetic adsorbent with enhanced mercury (II) adsorption property. Chem. Eng. J. 2015, 281, 468–481. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Zhou, X.; Bai, R. Removal of mercury (II) from aqueous solution with three commercial raw activated carbons. Res. Chem. Intermed. 2017, 43, 2273–2297. [Google Scholar] [CrossRef]
- Kerndorff, H.; Schnitzer, M. Sorption of metals on humic acid. Geochim. Cosmochim. Acta 1980, 44, 1701–1708. [Google Scholar] [CrossRef]

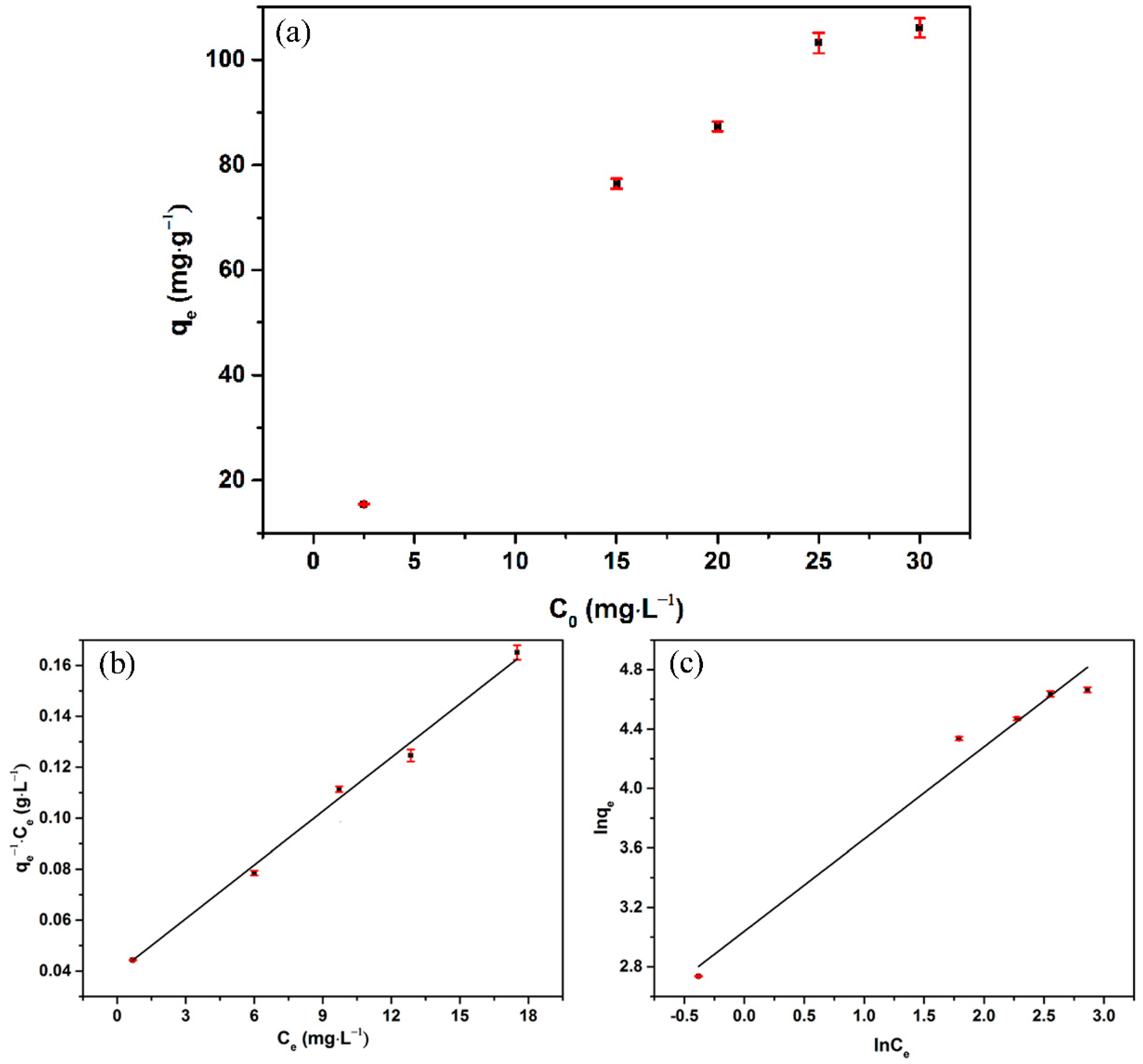
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| b (L·mg−1) | qmax (mg·g−1) | R2 | kf (L·mg−1) | n | R2 |
| 0.18 | 141.57 | 0.991 | 19.81 | 1.55 | 0.968 |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|
| k1 (min−1) | qe (mg·g−1) | R2 | k2 (g·mg−1·min−1) | qe (mg·g−1) | R2 |
| 0.1225 | 86.52 | 0.951 | 1.98 × 10−3 | 90.45 | 0.998 |
| Adsorbent | Equilibration Time (h) | qmax (mg·g−1) | Reference |
|---|---|---|---|
| Thiol-functionalized magnetic activated carbon | ~4 h | 366.3 | [47] |
| Lewatit MP 62 | 2 h | 151.5 | [48] |
| Purolite S 920 | 2 h | 29.2 | [48] |
| Thiol-modified Fe3O4@SiO2 | 4 h | 148.8 | [46] |
| Glutamic acid-modified cellulose fibrous | 10 min | 44.9 | [32] |
| Carbon nanotube | ~90 min | 100 | [49] |
| Coal-based activated carbon | ~3 h | 48.0 | [50] |
| HA/Fe3O4/OS | ~1 h | 141.6 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Qu, J.; Yu, Z.; Chen, D.; Su, T.; He, L.; Zhao, Z.; Zhou, C.; Hong, P.; Li, Y.; et al. Preparation of Micro-Nano Material Composed of Oyster Shell/Fe3O4 Nanoparticles/Humic Acid and Its Application in Selective Removal of Hg(II). Nanomaterials 2019, 9, 953. https://doi.org/10.3390/nano9070953
He C, Qu J, Yu Z, Chen D, Su T, He L, Zhao Z, Zhou C, Hong P, Li Y, et al. Preparation of Micro-Nano Material Composed of Oyster Shell/Fe3O4 Nanoparticles/Humic Acid and Its Application in Selective Removal of Hg(II). Nanomaterials. 2019; 9(7):953. https://doi.org/10.3390/nano9070953
Chicago/Turabian StyleHe, Chuxian, Junhao Qu, Zihua Yu, Daihuan Chen, Tiantian Su, Lei He, Zike Zhao, Chunxia Zhou, Pengzhi Hong, Yong Li, and et al. 2019. "Preparation of Micro-Nano Material Composed of Oyster Shell/Fe3O4 Nanoparticles/Humic Acid and Its Application in Selective Removal of Hg(II)" Nanomaterials 9, no. 7: 953. https://doi.org/10.3390/nano9070953






