Ag3PO4 Deposited on CuBi2O4 to Construct Z-Scheme Photocatalyst with Excellent Visible-Light Catalytic Performance Toward the Degradation of Diclofenac Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CuBi2O4/Ag3PO4
2.1.1. Synthesis of CuBi2O4
2.1.2. Synthesis of CuBi2O4/Ag3PO4
2.2. Characterization of Photocatalysts
2.3. Experiments of Photocatalytic Performance
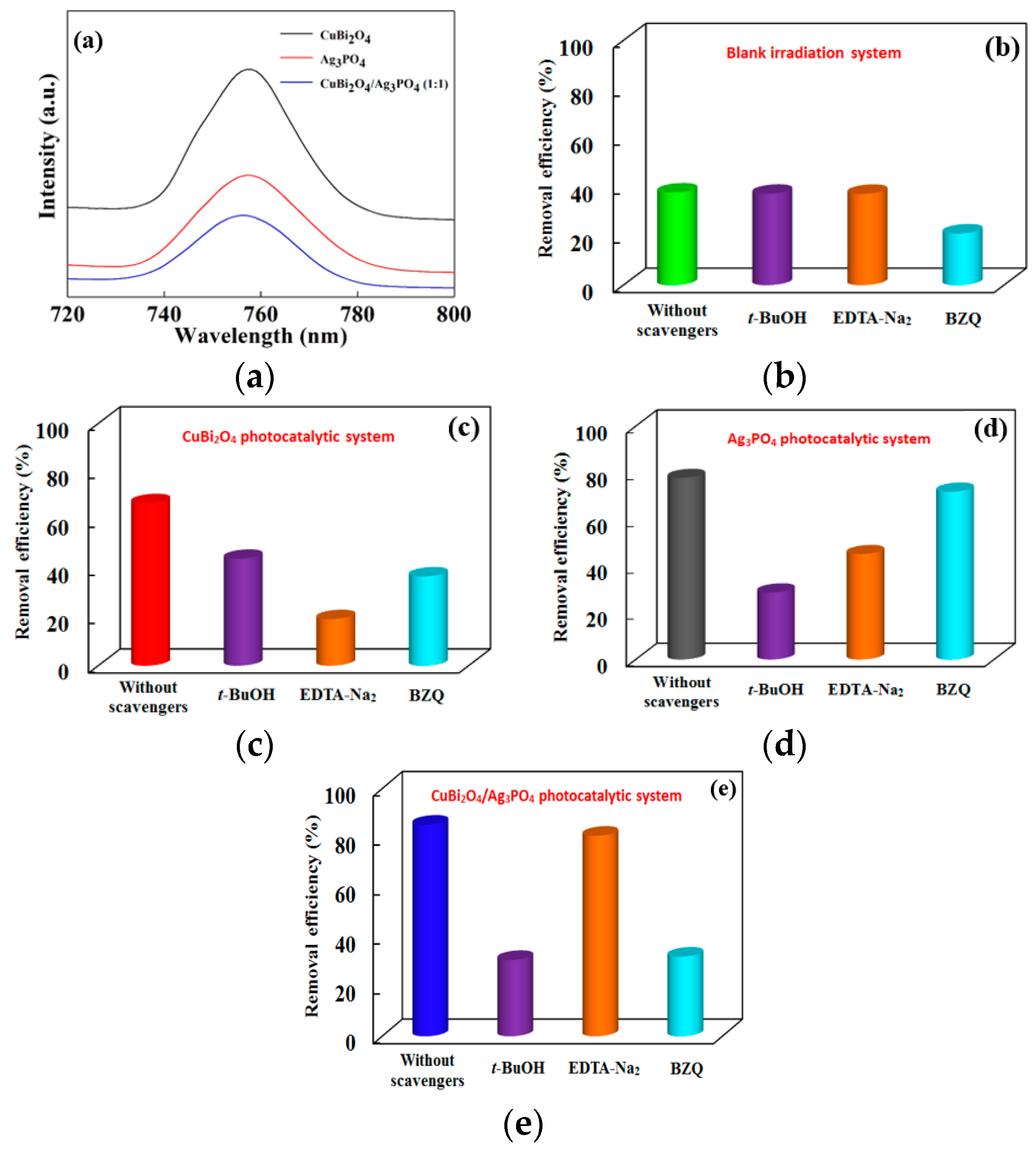
2.4. Analysis of Reactive Species
3. Results and Discussion
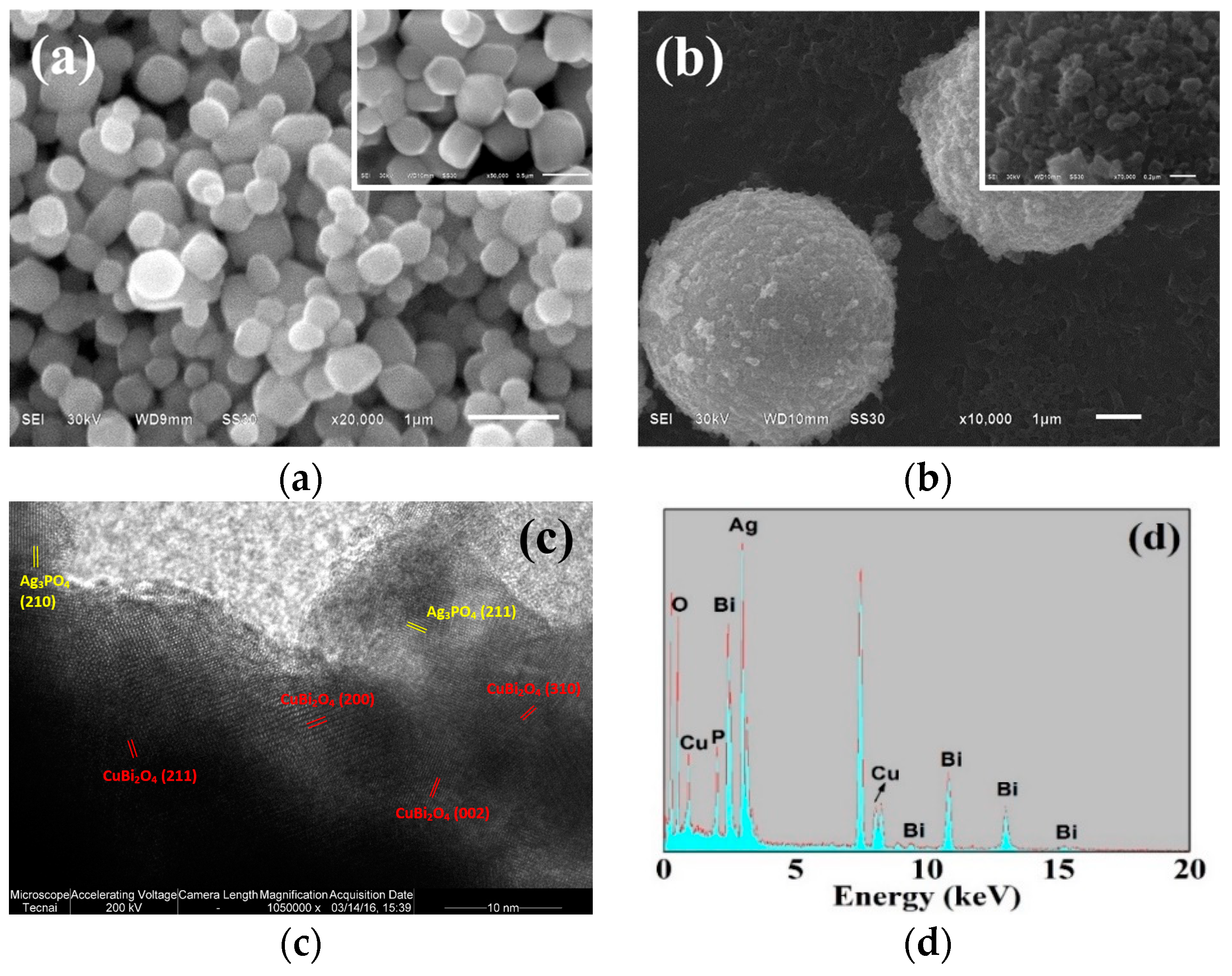
3.1. Morphology and Associated Formation Mechanism of CuBi2O4
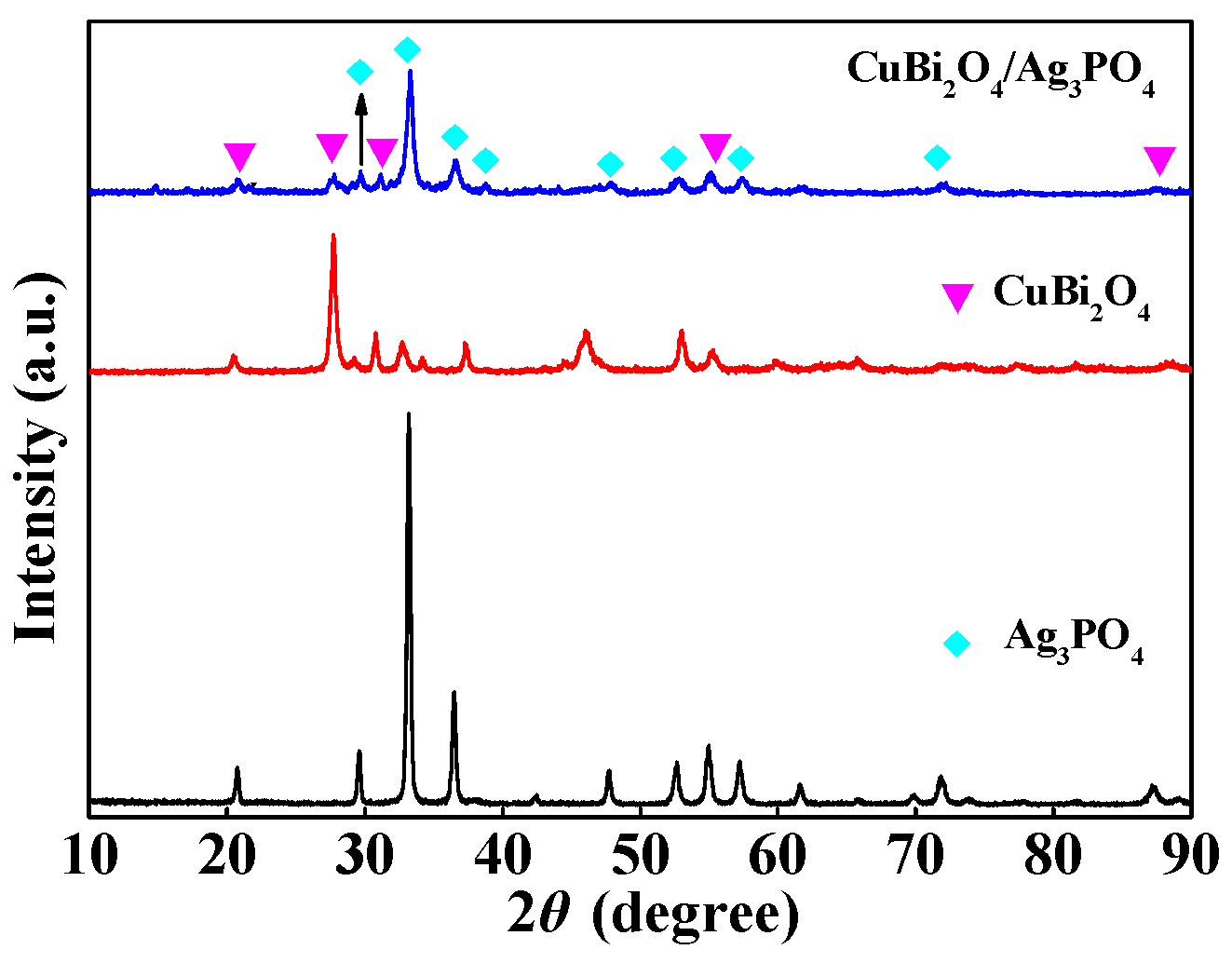
3.2. Characterization of CuBi2O4/Ag3PO4
3.2.1. Morphology
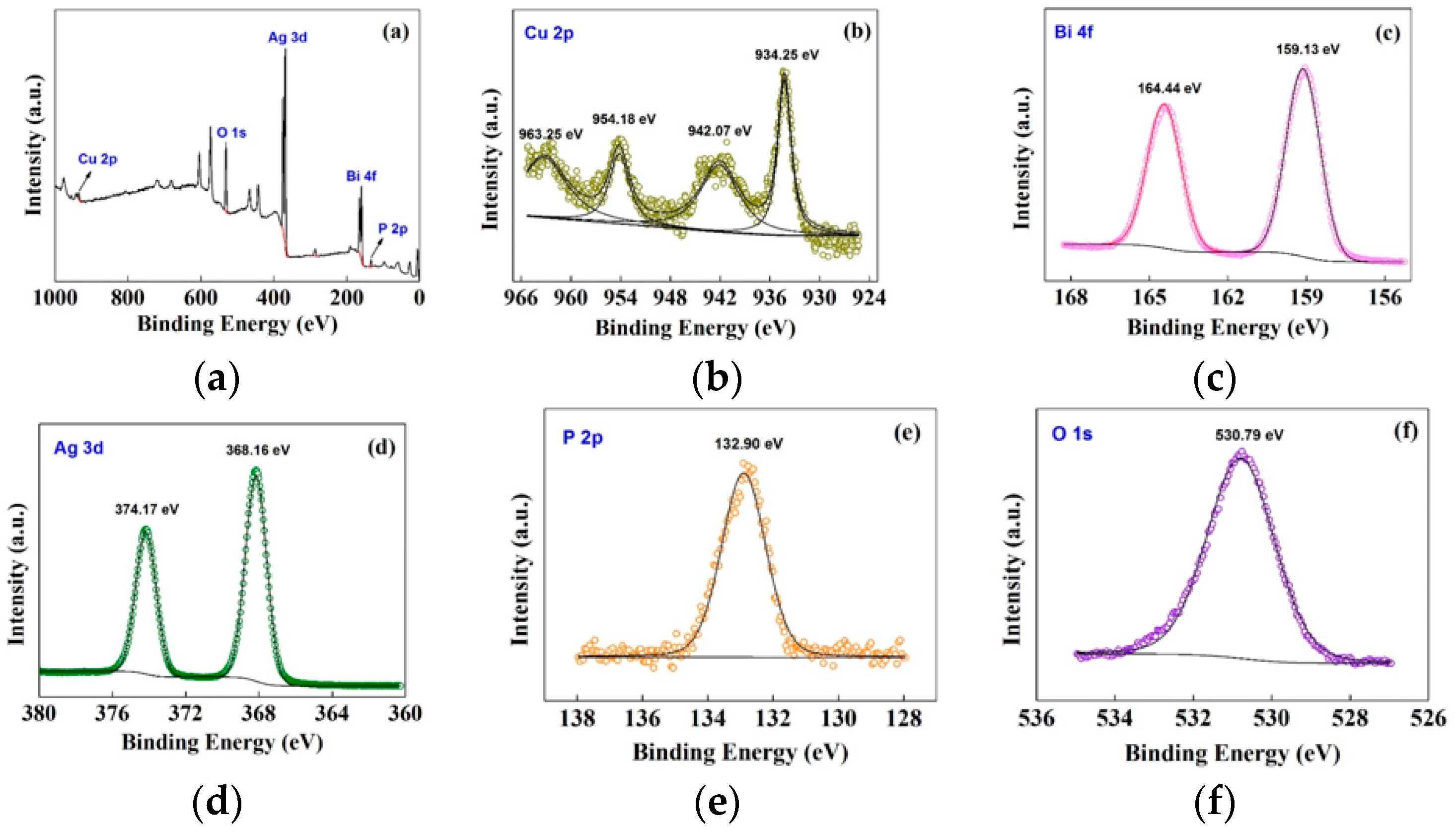
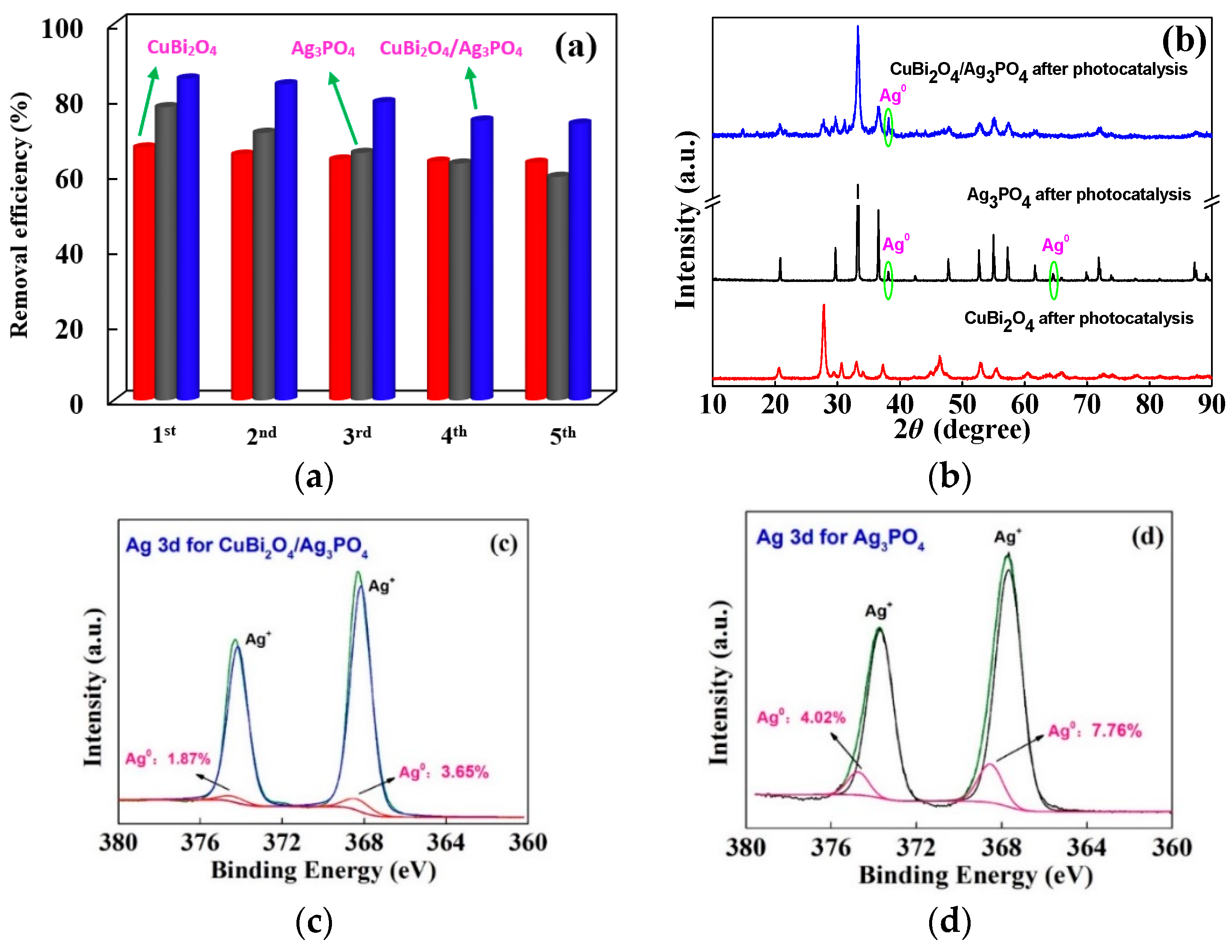
3.2.2. Component and Surface Property
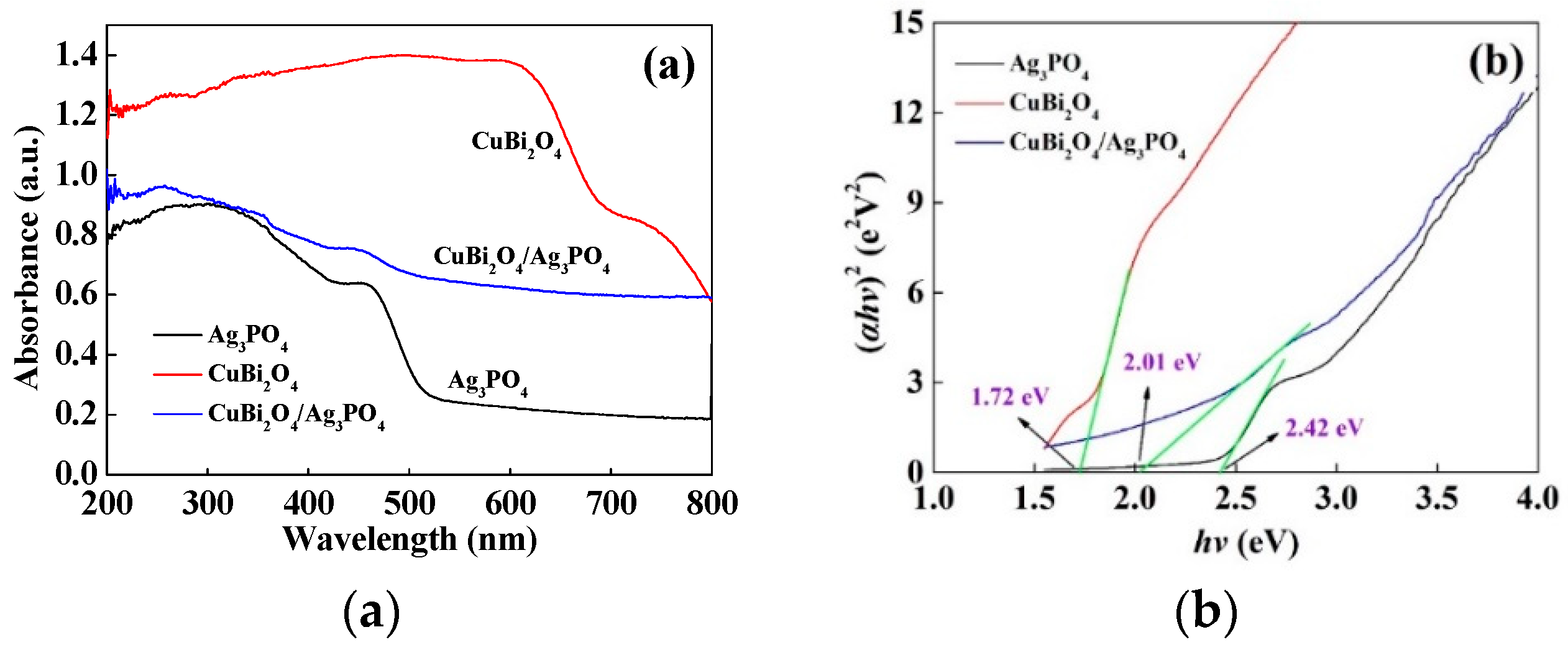
3.2.3. Optical Absorption Property
3.3. Photodegradation Performance of DS in Different Catalysts Systems
3.4. Photocatalytic Mechanism of Different Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, K.; Shi, Y.; Wang, X.; Li, Z. Sorption and retention of diclofenac on zeolite in the presence of cationic surfactant. J. Hazard. Mater. 2016, 323, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, D.; García-Villén, F.; Majdoub, H.; Zayani, M.B.; Viseras, C. Complex of chitosan pectin and clay as diclofenac carrier. Appl. Clay Sci. 2019, 172, 155–164. [Google Scholar] [CrossRef]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the Uptake Processes of Wastewater-Borne Pharmaceuticals by Vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Espino-Estévez, M.R.; Fernández-Rodríguez, C.; González-Díaz, O.M.; Araña, J.; Espinós, J.P.; Ortega-Méndez, J.A.; Doña-Rodríguez, J.M. Effect of TiO2-Pd and TiO2-Ag on the photocatalytic oxidation of diclofenac, isoproturon and phenol. Chem. Eng. J. 2016, 298, 82–95. [Google Scholar] [CrossRef]
- Bouju, H.; Nastold, P.; Beck, B.; Hollender, J.; Corvini, P.F.X.; Wintgens, T. Elucidation of biotransformation of diclofenac and 4′hydroxydiclofenac during biological wastewater treatment. J. Hazard. Mater. 2016, 301, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Xie, Y.; Ni, T.; Liu, G. Oxidative removal of diclofenac by chlorine dioxide: Reaction kinetics and mechanism. Chem. Eng. J. 2015, 279, 409–415. [Google Scholar] [CrossRef]
- Kohay, H.; Izbitski, A.; Mishael, Y.G. Developing polycation-clay sorbents for efficient filtration of diclofenac: Effect of dissolved organic matter and comparison to activated carbon. Environ. Sci. Technol. 2015, 49, 9280–9288. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation. Appl. Catal. B Environ. 2013, 134, 93–102. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Pan, Z.; Ma, S.; Li, L. Effective mineralization of Diclofenac by catalytic ozonation using Fe-MCM-41 catalyst. Chem. Eng. J. 2016, 304, 594–601. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. ChemInform Abstract: Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Buama, S.; Junsukhon, A.; Ngaotrakanwiwat, P.; Rangsunvigit, P. Validation of energy storage of TiO2NiO/TiO2 film by electrochemical process and photocatalytic activity. Chem. Eng. J. 2017, 309, 866–872. [Google Scholar] [CrossRef]
- Umer, M.; Tahir, M.; Azam, M.U.; Tahir, B.; Jaffar, M.M.; Alias, H. Montmorillonite dispersed single wall carbon nanotubes (SWCNTs)/TiO2 heterojunction composite for enhanced dynamic photocatalytic H2 production under visible light. Appl. Clay Sci. 2019, 174, 110–119. [Google Scholar] [CrossRef]
- Yang, Z.M.; Huang, G.F.; Huang, W.Q.; Wei, J.M.; Yan, X.G.; Liu, Y.Y.; Jiao, C.; Wan, Z.; Pan, A. Novel Ag3PO4/CeO2 composite with high efficiency and stability for photocatalytic applications. J. Mater. Chem. A 2014, 2, 1750–1756. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Q.; Dai, Q.; Wang, X. Fe doped CeO2 nanosheets for catalytic oxidation of 1, 2-dichloroethane: Effect of preparation method. Chem. Eng. J. 2016, 307, 1037–1046. [Google Scholar] [CrossRef]
- Liu, M.; Hou, L.A.; Xi, B.D.; Li, Q.; Hu, X.; Yu, S. Magnetically separable Ag/AgCl-zero valent iron particles modified zeolite X heterogeneous photocatalysts for tetracycline degradation under visible light. Chem. Eng. J. 2016, 302, 475–484. [Google Scholar] [CrossRef]
- Ye, Z.; Li, J.; Zhou, M.; Wang, H.; Ma, Y.; Huo, P.; Yu, L.; Yan, Y. Well-dispersed nebula-like ZnO/CeO2@HNTs heterostructure for efficient photocatalytic degradation of tetracycline. Chem. Eng. J. 2016, 304, 917–933. [Google Scholar] [CrossRef]
- Papoulis, D. Halloysite based nanocomposites and photocatalysis: A Review. Appl. Clay Sci. 2019, 168, 164–174. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Zhang, Y.; Yu, H.; Lu, G.; Ye, J.; Bi, Y. Concave trisoctahedral Ag3PO4 microcrystals with high-index facets and enhanced photocatalytic properties. Chem. Commun. 2013, 49, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jiao, Z.; Jin, B.; Feng, C.; Lu, G.; Bi, Y. Facile synthesis of porous Ag3PO4 photocatalysts with high self-stability and activity. RSC Adv. 2016, 6, 56166–56169. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Song, L. Super-high activity of Bi3+ doped Ag3PO4 and enhanced photocatalytic mechanism. Appl. Catal. B Environ. 2014, 152–153, 129–139. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Song, L. Co(II)-grafted Ag3PO4 photocatalysts with unexpected photocatalytic ability: Enhanced photogenerated charge separation efficiency, photocatalytic mechanism and activity. J. Hazard. Mater. 2015, 293, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Eswar, K.R.; Katkar, V.V.; Ramamurthy, P.C.; Madras, G. Novel AgBr/Ag3PO4 Decorated Ceria Nanoflake Composites for Enhanced Photocatalytic Activity toward Dyes and Bacteria under Visible Light. Ind. Eng. Chem. Res. 2015, 54, 8031–8042. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Zhou, H.; Kako, T.; Ye, J. Ag3PO4/In(OH)3 Composite Photocatalysts with Adjustable Surface-Electric Property for Efficient Photodegradation of Organic Dyes under Simulated Solar-Light Irradiation. J. Phys. Chem. C 2013, 117, 17716–17724. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Yang, J.; Zhang, S. Enhanced photocatalytic activity of Ag3PO4 photocatalyst via glucose-based carbonsphere modification. Chem. Eng. J. 2017, 309, 222–229. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, W.J.; Jiang, H. Facile synthesis of Ag/Ag3PO4/AMB composite with improved photocatalytic performance. Chem. Eng. J. 2017, 308, 889–896. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, D.; Liu, X.; Tang, W.; Wan, H.; Xiong, H.; Ma, R. Facile synthesis and characterization of core-shell structured Ag3PO4@Hal nanocomposites for enhanced photocatalytic properties. Appl. Clay Sci. 2017, 141, 132–137. [Google Scholar] [CrossRef]
- Cao, D.; Nasori, N.; Wang, Z.; Mi, Y.; Wen, L.; Yang, Y.; Qu, S.; Wang, Z.; Lei, Y. p-Type CuBi2O4: An easily accessible photocathodic material for high-efficiency water splitting. J. Mater. Chem. A 2016, 4, 8995–9001. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.; Lim, T.T. Rational design of hierarchically-structured CuBi2O4 composites by deliberate manipulation of the nucleation and growth kinetics of CuBi2O4 for environmental applications. Nanoscale 2016, 8, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Dai, Y.Z.; Guo, J.; Liu, T.H.; Wang, X.Y. Novel Magnetically Separable Reduced Graphene Oxide (RGO)/ZnFe2O4/Ag3PO4 Nanocomposites for Enhanced Photocatalytic Performance toward 2,4-Dichlorophenol under Visible Light. Ind. Eng. Chem. Res. 2015, 55, 568–578. [Google Scholar] [CrossRef]
- Wang, M. N-type hedgehog-like CuBi2O4 hierarchical microspheres: Room temperature synthesis and their photoelectrochemical properties. CrystEngComm 2015, 17, 4019–4025. [Google Scholar] [CrossRef]
- Oh, W.D.; Lua, S.K.; Dong, Z.; Lim, T.T. A novel three-dimensional spherical CuBi2O4 nanocolumn arrays with persulfate and peroxymonosulfate activation functionalities for 1H-benzotriazole removal. Nanoscale 2015, 7, 8149–8158. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Kelkar, S.; Naphade, R.; Ogale, S. Low temperature grown CuBi2O4 with flower morphology and its composite with CuO nanosheets for photoelectrochemical water splitting. J. Mater. Chem. A 2014, 2, 3661–3668. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Yang, G.; Liu, C.; Wang, J. Hydrothermal synthesis of CuBi2O4 nanosheets and their photocatalytic behavior under visible light irradiation. Mater. Lett. 2013, 107, 291–294. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Huang, H.; Liu, Y.; Kang, Z. Ag3PO4/SnO2 semiconductor nanocomposites with enhanced photocatalytic activity and stability. New J. Chem. 2012, 36, 1541–1544. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Li, J.; Bai, T.; Wang, J. Photocatalytic activity and adsorption performance of p-CuBi2O4/n-TiO2 p-n heterojunction composites prepared by in situ sol-gel coating method. J. Sol-Gel Sci. Technol. 2014, 71, 38–42. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Karthikeyan, K.; Kalpana, D.; Lee, Y.S.; Selvan, R.K. Surfactant-free hydrothermal synthesis of hierarchically structured spherical CuBi2O4 as negative electrodes for Li-ion hybrid capacitors. J. Colloid Interface Sci. 2016, 469, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.A.; Dao, C.D.; Hoang, T.T.T.; Dang, P.T.; Tran, H.T.K.; Nguyen, K.T.; Le, G.H.; Nguyen, T.V.; Lee, G.D. Synthesis of novel silver vanadates with high photocatalytic and antibacterial activities. Mater. Lett. 2014, 123, 176–180. [Google Scholar] [CrossRef]
- Teng, W.; Li, X.; Zhao, Q.; Chen, G. Fabrication of Ag/Ag3PO4/TiO2 heterostructure photoelectrodes for efficient decomposition of 2-chlorophenol under visible light irradiation. J. Mater. Chem. A 2013, 1, 9060–9068. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zhang, W.D. Morphology-controlled synthesis of Ag3PO4 microcrystals for high performance photocatalysis. CrystEngComm 2013, 27, 5407–5411. [Google Scholar] [CrossRef]
- Elaziouti, A.; Laouedj, N.; Bekka, A. Synergetic effects of Sr-doped CuBi2O4 catalyst with enhanced photoactivity under UVA-light irradiation. Environ. Sci. Pollut. Res. 2015, 23, 15862–15876. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Gao, Z.D.; Han, K.; Liu, Y.; Song, Y.Y. Synthesis of Magnetically Separable Ag3PO4/TiO2/Fe3O4 Heterostructure with Enhanced Photocatalytic Performance under Visible Light for Photoinactivation of Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 15122–15131. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Na, Z.; Zhao, Y.; Jing, L.; Lu, L. Sunlight-Assisted Degradation of Dye Pollutants in Ag3PO4 Suspension. Ind. Eng. Chem. Res. 2012, 51, 5167–5173. [Google Scholar]
- Patil, R. Low temperature grown CuBi2O4 with flower morphology and its composite with CuO nanosheets for photoelectrochemical water splitting. Bioorg. Med. Chem. Lett. 2014, 19, 92–95. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, G.; Liu, H.; Wang, Y.; He, Z.; Wang, G. Diclofenac photodegradation under simulated sunlight: Effect of different forms of nitrogen and kinetics. J. Hazard. Mater. 2011, 192, 411–418. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Ag3PO4/ZnO: An efficient visible-light-sensitized composite with its application in photocatalytic degradation of Rhodamine B. Mater. Res. Bull. 2013, 48, 106–113. [Google Scholar] [CrossRef]



| Catalyst | Specific Surface Area (m2/g) | Kapp (min−1) | R2 |
|---|---|---|---|
| Blank irradiation | / | 0.0041 | 0.87 |
| CuBi2O4 | 2.307 | 0.0084 | 0.95 |
| Ag3PO4 | 3.874 | 0.0112 | 0.89 |
| Commercial TiO2 | / | 0.0069 | 0.94 |
| CuBi2O4/Ag3PO4 (2:1) | 4.938 | 0.0098 | 0.92 |
| CuBi2O4/Ag3PO4 (1:1) | 7.846 | 0.0143 | 0.91 |
| CuBi2O4/Ag3PO4 (1:2) | 7.442 | 0.0138 | 0.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yu, C.; Zhu, R.; Li, N.; Chen, J.; Li, S.; Xia, W.; Xu, S.; Wang, H.; Chen, X. Ag3PO4 Deposited on CuBi2O4 to Construct Z-Scheme Photocatalyst with Excellent Visible-Light Catalytic Performance Toward the Degradation of Diclofenac Sodium. Nanomaterials 2019, 9, 959. https://doi.org/10.3390/nano9070959
Chen X, Yu C, Zhu R, Li N, Chen J, Li S, Xia W, Xu S, Wang H, Chen X. Ag3PO4 Deposited on CuBi2O4 to Construct Z-Scheme Photocatalyst with Excellent Visible-Light Catalytic Performance Toward the Degradation of Diclofenac Sodium. Nanomaterials. 2019; 9(7):959. https://doi.org/10.3390/nano9070959
Chicago/Turabian StyleChen, Xiaojuan, Chunmu Yu, Runliang Zhu, Ning Li, Jieming Chen, Shuai Li, Wei Xia, Song Xu, Hailong Wang, and Xin Chen. 2019. "Ag3PO4 Deposited on CuBi2O4 to Construct Z-Scheme Photocatalyst with Excellent Visible-Light Catalytic Performance Toward the Degradation of Diclofenac Sodium" Nanomaterials 9, no. 7: 959. https://doi.org/10.3390/nano9070959





