N, S Dual-Doped Carbon Derived from Dye Sludge by Using Polymeric Flocculant as Soft Template
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
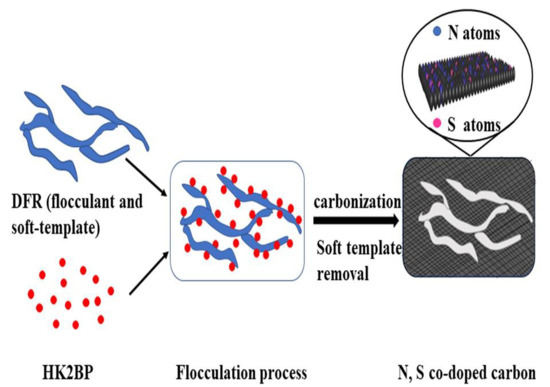
2.2. Azo Dye Flocculation and Sludge Flocs Preparation
2.3. Synthesis of N, S Dual-Doped Carbon Materials N, S-DF-x
2.4. Materials Characterization
2.5. Electrochemical Characterization
2.5.1. ORR Measurements
2.5.2. Capacitive Measurements
3. Results and Discussion
3.1. Preparation and Composition of Flocculation Sludge
3.2. Characterization of N, S-DF-x.
3.3. Electrochemical Performance of N, S-DF-x
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Jin, X.-C.; Liu, G.-Q.; Xu, Z.-H.; Tao, W.-Y. Decolorization of a Dye Industry Effluent by Aspergillus Fumigatus Xc6. Appl. Microbiol. Biotechnol. 2006, 74, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.W.; Wang, H.; Yang, J.; Niu, Z.; Lu, H.; Yang, Y.; Cheng, L.; Guo, L. Dye Wastewater Cleanup by Graphene Composite Paper for Tailorable Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 21298–21306. [Google Scholar] [CrossRef]
- Asad, S.; Amoozegar, M.A.; Pourbabaee, A.A.; Sarbolouki, M.N.; Dastgheib, S.M. Decolorization of Textile Azo Dyes by Newly Isolated Halophilic and Halotolerant Bacteria. Bioresour. Technol. 2007, 98, 2082–2088. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Facile Synthesis of Sewage Sludge-Derived in-Situ Multi-Doped Nanoporous Carbon Material for Electrocatalytic Oxygen Reduction. Sci. Rep. 2016, 6, 27570. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Tak, J.K.; Holt, C.M.B.; Tan, X.; Xu, Z.; Amirkhiz, B.S.; Harfield, D.; Anyia, A.; Stephenson, T.; et al. Supercapacitors Based on Carbons with Tuned Porosity Derived from Paper Pulp Mill Sludge Biowaste. Carbon 2013, 57, 317–328. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Khaled, P.S.; Shubin, Y.; Yenny, H.; Andreas, W.; Andrey, T.; Xinliang, F.; Klaus, M. Nitrogen-Doped Graphene and Its Iron-Based Composite as Efficient Electrocatalysts for Oxygen Reduction Reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar]
- Xiang, Z.; Cao, D.; Huang, L.; Shui, J.; Wang, M.; Dai, L. Nitrogen-Doped Holey Graphitic Carbon from 2D Covalent Organic Polymers for Oxygen Reduction. Adv. Mater. 2014, 26, 3315–3320. [Google Scholar] [CrossRef]
- Wen, Y.; Tim-Patrick, F.; Markus, A. Efficient Metal-Free Oxygen Reduction in Alkaline Medium on High-Surface-Area Mesoporous Nitrogen-Doped Carbons Made from Ionic Liquids and Nucleobases. J. Am. Chem. Soc. 2011, 133, 206–209. [Google Scholar]
- Taeyoung, K.; Gyujin, J.; Seonmi, Y.; Suh, K.S.; Ruoff, R.S. Activated Graphene-Based Carbons as Supercapacitor Electrodes with Macro- and Mesopores. ACS Nano 2013, 7, 6899–6905. [Google Scholar]
- Zhao, Z.; Li, M.; Zhang, L.; Dai, L.; Xia, Z. Design Principles for Heteroatom-Doped Carbon Nanomaterials as Highly Efficient Catalysts for Fuel Cells and Metal-Air Batteries. Adv. Mater. 2015, 27, 6834–6840. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and Sulfur Co-Doped Porous Carbon Nanosheets Derived from Willow Catkin for Supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, X.; Huang, J.; Han, J.; Zhang, Z.; Han, X.; Xu, P.; Song, B. S, N Dual-Doped Graphene-Like Carbon Nanosheets as Efficient Oxygen Reduction Reaction Electrocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhu, D.; Liu, M.; Duan, H.; Wang, Z.; Lv, Y.; Xiong, W.; Zhu, Q.; Li, L.; Chai, X.; et al. Cooking Carbon with Protic Salt: Nitrogen and Sulfur Self-Doped Porous Carbon Nanosheets for Supercapacitors. Chem. Eng. J. 2018, 347, 233–242. [Google Scholar] [CrossRef]
- Xu, G.; Han, J.; Ding, B.; Nie, P.; Pan, J.; Dou, H.; Li, H.; Zhang, X. Biomass-Derived Porous Carbon Materials with Sulfur and Nitrogen Dual-Doping for Energy Storage. Green Chem. 2015, 17, 1668–1674. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Xiao, X.; Shao, J.; Wei, J.; Pang, H.; Yu, Y. N, S Co-Doped 3D Mesoporous Carbon–Co3Si2O5(OH)4 Architectures for High-Performance Flexible Pseudo-Solid-State Supercapacitors. J. Mater. Chem. A 2017, 5, 12774–12781. [Google Scholar] [CrossRef]
- Si, W.; Zhou, J.; Zhang, S.; Li, S.; Xing, W.; Zhuo, S. Tunable N-Doped or Dual N, S-Doped Activated Hydrothermal Carbons Derived from Human Hair and Glucose for Supercapacitor Applications. Electrochim. Acta 2013, 107, 397–405. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Wu, D.; Zhao, X.; Zhou, Z. S-Doped N-Rich Carbon Nanosheets with Expanded Interlayer Distance as Anode Materials for Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1604108. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene Oxide-Polydopamine Derived N, S-Codoped Carbon Nanosheets as Superior Bifunctional Electrocatalysts for Oxygen Reduction and Evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xu, M.; Wu, L.; Jia, X.; Wei, T.; Zhang, S.; Guo, X. Smart Flocculant with Temperature and Ph Response Derived from Starch. Rsc. Adv. 2016, 6, 44383–44391. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.G.; Dai, S. Soft-Templated Mesoporous Carbon-Carbon Nanotube Composites for High Performance Lithium-Ion Batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Meng, X.-L.; Nie, Y.; Sun, J.; Cheng, W.-G.; Wang, J.-Q.; He, H.-Y.; Zhang, S.-J. Functionalized Dicyandiamide–Formaldehyde Polymers as Efficient Heterogeneous Catalysts for Conversion of CO2 into Organic Carbonates. Green Chem. 2014, 16, 2771–2778. [Google Scholar] [CrossRef]
- Zhong, H.; Gong, X.; Zhang, S.; Tang, P.; Li, D.; Feng, Y. Design and Synthesis of Cobalt-Based Electrocatalysts for Oxygen Reduction Reaction. Chem. Rec. 2018, 18, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Dai, P.; Jiang, X.; Wang, X.; Zhang, C.; Tang, D.; Weng, Q.; Wang, X.; Pakdel, A.; Tang, C.; et al. Template-Free Synthesis of Boron Nitride Foam-Like Porous Monoliths and Their High-End Applications in Water Purification. J. Mater. Chem. A 2016, 4, 1469–1478. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Zhang, Z.; Lei, Z. Facile Synthesis of Poly(P-Phenylenediamine)-Derived Three-Dimensional Porous Nitrogen-Doped Carbon Networks for High Performance Supercapacitors. J. Phys. Chem. C 2014, 118, 29507–29516. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Zhou, D.; Niu, H.; Jin, Q.; Qu, F. Synthesis of Mesoporous Graphitic Carbon Fibers with High Performance for Supercapacitor. Electrochim. Acta 2015, 159, 116–123. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-Doped Porous Carbon Derived from Biomass Waste for High-Performance Supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Niu, H.; Li, Z.; Du, Y.; Cizek, P.; Xie, Z.; Xiong, H.; Lin, T. High-Performance Supercapacitor Electrode Materials from Cellulose-Derived Carbon Nanofibers. ACS Appl. Mater. Interfaces 2015, 7, 14946–14953. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hu, L.; Li, J.; Wang, E. Recent Advancements in Transition Metal-Nitrogen-Carbon Catalysts for Oxygen Reduction Reaction. Electroanalysis 2018, 30, 1217–1228. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, e1703691. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Yang, L.; Zhang, Z.; Xi, X. Honeysuckle-Derived Hierarchical Porous Nitrogen, Sulfur, Dual-Doped Carbon for Ultra-High Rate Lithium Ion Battery Anodes. J. Power Sources 2016, 333, 193–202. [Google Scholar] [CrossRef]
- Xin, G.; Wang, Y.; Jia, S.; Tian, P.; Zhou, S.; Zang, J. Synthesis of Nitrogen-Doped Mesoporous Carbon from Polyaniline with an F127 Template for High-Performance Supercapacitors. Appl. Surf. Sci. 2017, 422, 654–660. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Woo, S.I.; Jung, Y. On the Mechanism of Enhanced Oxygen Reduction Reaction in Nitrogen-Doped Graphene Nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, L.; Cao, D. Nitrogen and Fluorine-Codoped Porous Carbons as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. ACS Appl. Mater. Interfaces 2017, 9, 32859–32867. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.D.; Zhou, Z.Y.; Lai, Y.J.; Rauf, M.; Wang, Y.; Pan, J.; Zhuang, L.; Wang, Q.; Wang, Y.C.; et al. Aminothiazole-Derived N, S,Fe-Doped Graphene Nanosheets as High Performance Electrocatalysts for Oxygen Reduction. Chem. Commun. 2015, 51, 17092–17095. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-Based Nitrogen Self-Doped Hierarchical Porous Carbon Aerogels Derived from Chitosan for High Performance Supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Lv, Y.K.; Gan, L.H.; Liu, M.X.; Xiong, W.; Xu, Z.J.; Zhu, D.Z.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, X.; Zhu, B.; Liu, P.-F.; Lu, H.-T. Micro-Mesoporous Carbon Spheres Derived from Carrageenan as Electrode Material for Supercapacitors. J. Power Sources 2014, 268, 584–590. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Song, P.; Pan, J.; Zhuang, L.; Xu, W.; Xing, W. Fluorine-Doped Carbon Blacks: Highly Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2013, 3, 1726–1729. [Google Scholar] [CrossRef]
- Yan, W.; Cao, X.; Tian, J.; Jin, C.; Ke, K.; Yang, R. Nitrogen/Sulfur Dual-Doped 3d Reduced Graphene Oxide Networks-Supported CoFe2O4 with Enhanced Electrocatalytic Activities for Oxygen Reduction and Evolution Reactions. Carbon 2016, 99, 195–202. [Google Scholar] [CrossRef]
- Ahn, S.H.; Manthiram, A. Self-Templated Synthesis of Co- and N-Doped Carbon Microtubes Composed of Hollow Nanospheres and Nanotubes for Efficient Oxygen Reduction Reaction. Small 2017, 13, 1603437. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, K.; Deng, L.; Liu, Y.-N. Protein Hydrogel Networks: A Unique Approach to Heteroatom Self-Doped Hierarchically Porous Carbon Structures as an Efficient Orr Electrocatalyst in Both Basic and Acidic Conditions. Appl. Catal. B 2019, 246, 89–99. [Google Scholar] [CrossRef]
- Wu, X.; Shi, Z.; Tjandra, R.; Cousins, A.J.; Sy, S.; Yu, A.; Berry, R.M.; Tam, K.C. Nitrogen-Enriched Porous Carbon Nanorods Templated by Cellulose Nanocrystals as High Performance Supercapacitor Electrodes. J. Mater. Chem. A 2015, 3, 23768–23777. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, R.N. Multifunctional Nanostructured Electrocatalysts for Energy Conversion and Storage: Current Status and Perspectives. Nanoscale 2018, 10, 11241–11280. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, N.; Lei, S.; Yan, R.; Tian, X.; Wang, J.; Song, Y.; Xu, D.; Guo, Q.; Liu, L. Promising Biomass-Based Activated Carbons Derived from Willow Catkins for High Performance Supercapacitors. Electrochim. Acta 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.D.; Yu, Q.; Wang, F.Y.; Zhu, H.L.; Zhang, R.L.; Liu, X. Nitrogen-Doped Hollow Carbon Spheres with a Wrinkled Surface: Their One-Pot Carbonization Synthesis and Supercapacitor Properties. Chem. Commun. 2016, 52, 11693–11696. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, D.; Wu, L.; Wei, T.; Liu, L.; Lv, Y.; Yu, F.; Chen, L.; Shi, Y. N, S Dual-Doped Carbon Derived from Dye Sludge by Using Polymeric Flocculant as Soft Template. Nanomaterials 2019, 9, 991. https://doi.org/10.3390/nano9070991
Luan D, Wu L, Wei T, Liu L, Lv Y, Yu F, Chen L, Shi Y. N, S Dual-Doped Carbon Derived from Dye Sludge by Using Polymeric Flocculant as Soft Template. Nanomaterials. 2019; 9(7):991. https://doi.org/10.3390/nano9070991
Chicago/Turabian StyleLuan, Daofeng, Liang Wu, Tingting Wei, Liu Liu, Yin Lv, Feng Yu, Long Chen, and Yulin Shi. 2019. "N, S Dual-Doped Carbon Derived from Dye Sludge by Using Polymeric Flocculant as Soft Template" Nanomaterials 9, no. 7: 991. https://doi.org/10.3390/nano9070991
APA StyleLuan, D., Wu, L., Wei, T., Liu, L., Lv, Y., Yu, F., Chen, L., & Shi, Y. (2019). N, S Dual-Doped Carbon Derived from Dye Sludge by Using Polymeric Flocculant as Soft Template. Nanomaterials, 9(7), 991. https://doi.org/10.3390/nano9070991