Controllable Synthesis of Zn-Doped α-Fe2O3 Nanowires for H2S Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe Source Solution
2.3. Synthesis of Zn-Doped α-Fe2O3 Nanowires
2.4. Characterizations
2.5. Fabrication and Measurement of Gas Sensor
3. Results and Discussion
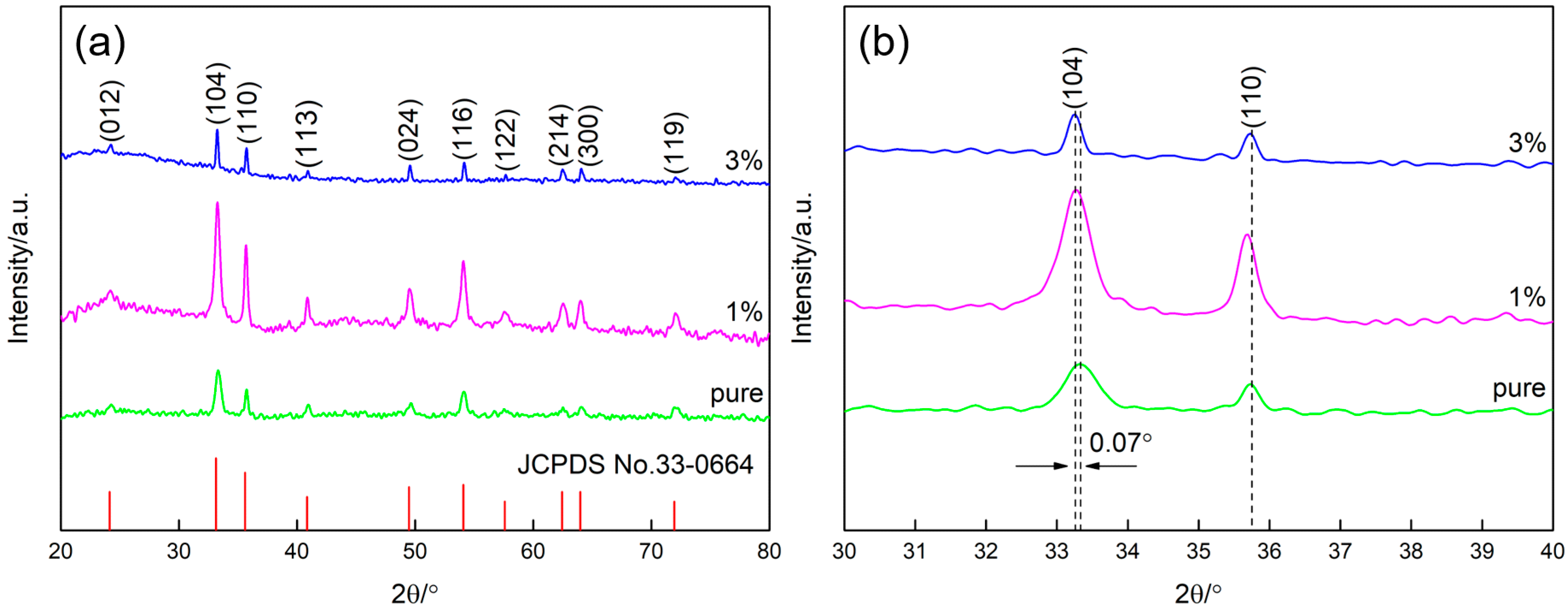
3.1. Structural and Morphology Characterization
3.2. Growth Mechanism
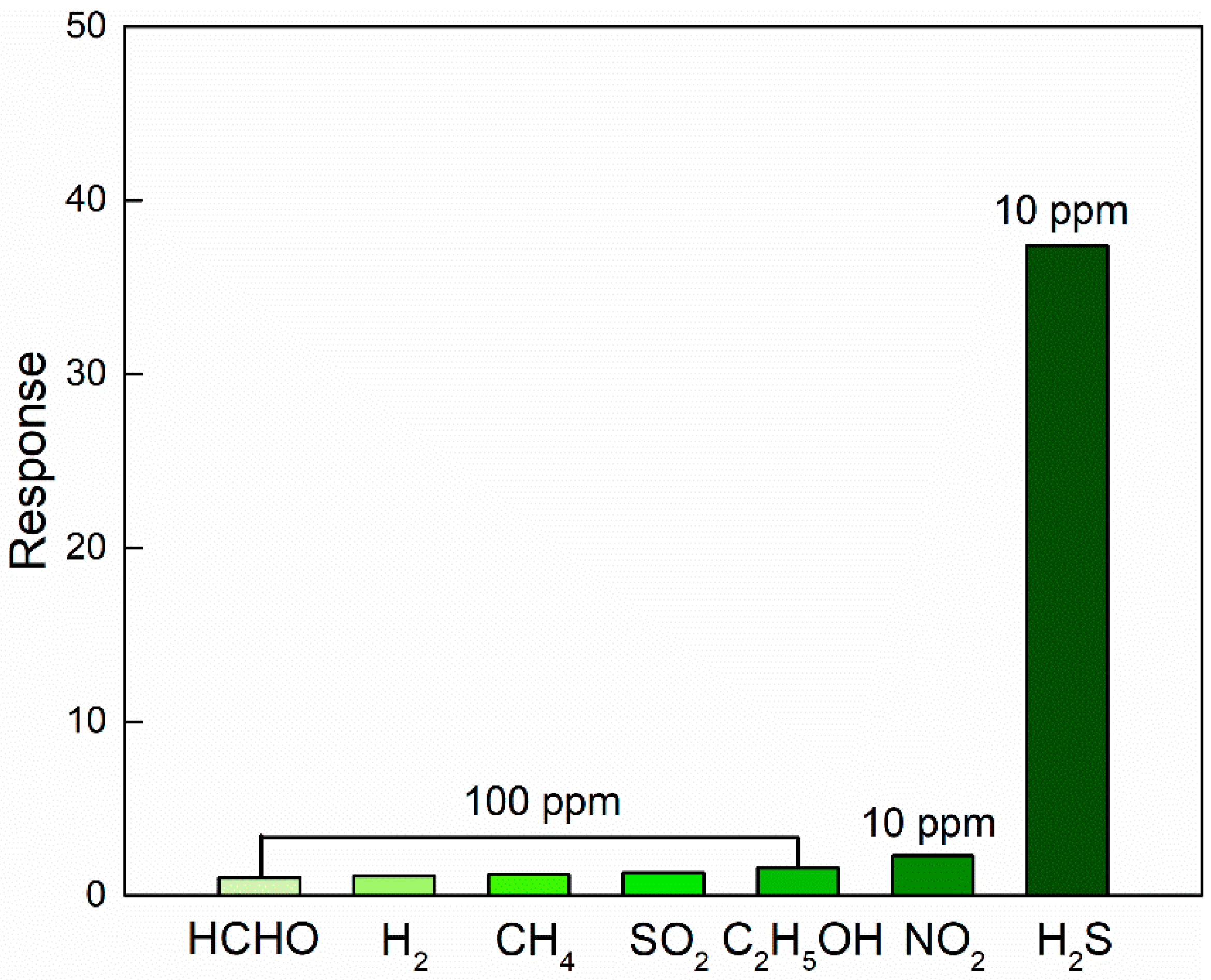
3.3. Gas Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, Z.; Luo, Y.; Duan, G. Self-assembly of Cu2O monolayer colloidal particle film allows the fabrication of CuO sensor with superselectivity for hydrogen sulfide. ACS Appl. Mater. Interfaces 2019, 11, 8164–8174. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Wang, Y.; Ma, Y.; Zhong, J.; Sun, X. Facile fabrication of a well-ordered porous Cu-doped SnO2 thin film for H2S sensing. ACS Appl. Mater. Interfaces 2014, 6, 14975–14980. [Google Scholar] [CrossRef]
- Tian, K.; Wang, X.; Yu, Z.; Li, H.; Guo, X. Hierarchical and hollow Fe2O3 nanoboxes derived from metal–organic frameworks with excellent sensitivity to H2S. ACS Appl. Mater. Interfaces 2017, 9, 29669–29676. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L.H. Hierarchical NiO cube/nitrogen-doped reduced graphene oxide composite with enhanced H2S sensing properties at low temperature. ACS Appl. Mater. Interfaces 2017, 9, 26293–26303. [Google Scholar] [CrossRef]
- Chen, Y.J.; Gao, X.M.; Di, X.P.; Ouyang, Q.Y.; Gao, P.; Qi, L.H.; Li, C.Y.; Zhu, C.L. Porous iron molybdate nanorods: In situ diffusion synthesis and low-temperature H2S gas sensing. ACS Appl. Mater. Interfaces 2013, 5, 3267–3274. [Google Scholar] [CrossRef]
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.K.; Gupta, S.K.; Yakhmi, J.V. Room-temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuators B Chem. 2008, 133, 456–461. [Google Scholar] [CrossRef]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.Q.; Wang, Z. Room-temperature high-performance H2S sensor based on porous CuO nanosheets prepared by hydrothermal method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Zhang, J.H.; Cai, G.F.; Zhou, D.; Tang, H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Co-doped NiO nanoflake array films with enhanced electrochromic properties. J. Mater. Chem. C 2014, 2, 7013–7021. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Sekiguchi, T.; Ohashi, N.; Sakaguchi, I.; Koumoto, K. Low-temperature fabrication of light-emitting zinc oxide micropatterns using self-assembled monolayers. Adv. Mater. 2002, 14, 418–421. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Qiao, W.M.; Shao, B.; Zhao, Q.K.; Zhang, L.J.; Fan, Z.J. Rapid microwave-assisted synthesis of graphene nanosheet/Co3O4 composite for supercapacitors. Electrochim. Acta 2010, 55, 6973–6978. [Google Scholar] [CrossRef]
- Ko, S.H.; Lee, D.; Kang, H.W.; Nam, K.H.; Yeo, J.Y.; Hong, S.J.; Grigoropoulos, C.P.; Sung, H.J. Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell. Nano. Lett. 2011, 11, 666–671. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Vijaya, J.J.; Jayakumar, C.; Maaza, M.; Jeyaraj, B. Photocatalytic degradation effect of malachite green and catalytic hydrogenation by UV–illuminated CeO2/CdO multilayered nanoplatelet arrays: Investigation of antifungal and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2017, 169, 110–123. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sens. Actuators B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Shen, Y.B.; Wang, W.; Chen, X.X.; Zhang, B.Q.; Wei, D.Z.; Gao, S.L.; Cui, B.Y. Nitrogen dioxide sensing using tungsten oxide microspheres with hierarchical nanorod-assembled architectures by a complexing surfactant-mediated hydrothermal route. J. Mater. Chem. A 2016, 4, 1345–1352. [Google Scholar] [CrossRef]
- Chen, X.X.; Shen, Y.B.; Zhou, P.F.; Zhao, S.K.; Zhong, X.X.; Li, T.T.; Han, C.; Wei, D.Z.; Meng, D. NO2 sensing properties of one-pot-synthesized ZnO nanowires with Pd functionalization. Sens. Actuators B Chem. 2019, 280, 151–161. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhong, X.; Han, C.; Zhao, Q.; Wei, D. Design of Au@WO3 core–shell structured nanospheres for ppb-level NO2 sensing. Sens. Actuators B Chem. 2019, 282, 917–926. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Deng, J.; Zhang, R.; Zhang, T. Ethanol gas detection using a yolk-shell (core-shell) α-Fe2O3 nanospheres as sensing material. ACS Appl. Mater. Interfaces 2015, 7, 13098–13104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 273, 1278–1290. [Google Scholar] [CrossRef]
- Modaberi, M.R.; Rooydell, R.; Brahma, S.; Akande, A.A.; Mwakikunga, B.W.; Liu, C.P. Enhanced response and selectivity of H2S sensing through controlled Ni doping into ZnO nanorods by using single metal organic precursors. Sens. Actuators B Chem. 2018, 273, 1278–1290. [Google Scholar] [CrossRef]
- Wang, C.X.; Zeng, W.; Luo, L.J.; Zhang, P.G.; Wang, Z.C. Gas-sensing properties and mechanisms of Cu-doped SnO2 spheres towards H2S. Ceram. Int. 2016, 42, 10006–10013. [Google Scholar] [CrossRef]
- Zhang, H.J.; Meng, F.N.; Liu, L.Z.; Chen, Y.J. Convenient route for synthesis of alpha-Fe2O3 and sensors for H2S gas. J. Alloy. Compd. 2019, 774, 1181–1188. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Zheng, Y.F.; Lee, J.H.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2019, 281, 453–461. [Google Scholar] [CrossRef]
- Shan, H.; Liu, C.; Liu, L.; Zhang, J.; Li, H.; Liu, Z.; Zhang, X.; Bo, X.; Chi, X. Excellent toluene sensing properties of SnO2–Fe2O3 interconnected nanotubes. ACS Appl. Mater. Interfaces 2013, 5, 6376–6380. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.B.; Bi, H.S.; Li, T.T.; Zhong, X.X.; Chen, X.X.; Fan, A.F.; Wei, D.Z. Low-temperature and highly enhanced NO2 sensing performance of Au-functionalized WO3 microspheres with a hierarchical nanostructure. Appl. Surf. Sci. 2018, 434, 922–931. [Google Scholar] [CrossRef]
- Shen, Y.B.; Chen, X.X.; Wang, W.; Gong, Y.; Chen, S.H.; Liu, J.T.; Wei, D.Z.; Meng, D.; San, X.G. Complexing surfactants-mediated hydrothermal synthesis of WO3 microspheres for gas sensing applications. Mater. Lett. 2016, 163, 150–153. [Google Scholar] [CrossRef]
- Shen, Y.B.; Wang, W.; Fan, A.F.; Wei, D.Z.; Liu, W.G.; Han, C.; Shen, Y.S.; Meng, D.; San, X.G. Highly sensitive hydrogen sensors based on SnO2 nanomaterials with different morphologies. Int. J. Hydrog. Energy 2015, 40, 15773–15779. [Google Scholar] [CrossRef]
- Deng, J.; Ma, J.; Mei, L.; Tang, Y.; Chen, Y.; Lv, T.; Xu, Z.; Wang, T. Porous α-Fe2O3 nanosphere-based H2S sensor with fast response, high selectivity and enhanced sensitivity. J. Mater. Chem. A 2013, 1, 12400–12403. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [PubMed]
- Li, Z.; Lin, Z.; Wang, N.; Huang, Y.; Kuang, Z.; Liu, W.; Fu, Y.; Zhang, Z. Facile synthesis of α-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater. Des. 2016, 110, 532–539. [Google Scholar] [CrossRef]
- Liu, C.; Gao, H.; Wang, L.; Wang, T.; Yang, X.; Sun, P.; Gao, Y.; Liang, X.; Liu, F.; Song, H.; et al. Facile synthesis and the enhanced sensing properties of Pt-loaded α-Fe2O3 porous nanospheres. Sens. Actuators B Chem. 2017, 252, 1153–1162. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, F.; Zhu, B.; Wang, S.; Wu, S.; Huang, W. Synthesis and characterization of Pd-doped α-Fe2O3 H2S sensor with low power consumption. Mater. Sci. Eng. B 2007, 140, 98–102. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Cao, J.; Kong, F.; Xia, H.; Zhang, J.; Zhu, B.; Wang, S.; Wu, S. Low-temperature H2S sensors based on Ag-doped α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2008, 131, 183–189. [Google Scholar] [CrossRef]
- Balouria, V.; Ramgir, N.S.; Singh, A.; Debnath, A.K.; Mahajan, A.; Bedi, R.K.; Aswal, D.K.; Gupta, S.K. Enhanced H2S sensing characteristics of Au modified Fe2O3 thin films. Sens. Actuators B Chem. 2015, 219, 125–132. [Google Scholar] [CrossRef]
- Giebelhaus, I.; Varechkina, E.; Fischer, T.; Rumyantseva, M.; Ivanov, V.; Gaskov, A.; Morante, J.R.; Arbiol, J.; Tyrra, W.; Mathur, S. One-dimensional CuO–SnO2 p–n heterojunctions for enhanced detection of H2S. J. Mater. Chem. 2013, 1, 11261–11268. [Google Scholar] [CrossRef]
- Chen, H.D.; Jin, K.L.; Wang, P.F.; Xu, J.C.; Han, Y.B.; Jin, H.X.; Jin, D.F.; Peng, X.L.; Hong, B.; Li, J.; et al. Highly enhanced gas-sensing properties of indium-doped mesoporous hematite nanowires. J. Phys. Chem. Solids 2018, 120, 271–278. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Cheng, M.; Gao, Y.; Zhao, L.; Li, S.; Liu, F.; Yan, X.; Zhang, T.; Sun, P.; et al. The preparation of reduced graphene oxide-encapsulated α-Fe2O3 hybrid and its outstanding NO2 gas sensing properties at room temperature. Sens. Actuators B Chem. 2018, 261, 252–263. [Google Scholar] [CrossRef]
- Chen, X.X.; Shen, Y.B.; Zhang, W.; Zhang, J.; Wei, D.Z.; Lu, R.; Zhu, L.J.; Li, H.S.; Shen, Y.S. In-situ growth of ZnO nanowire arrays on the sensing electrode via a facile hydrothermal route for high-performance NO2 sensor. Appl. Surf. Sci. 2018, 435, 1096–1104. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, S.F.; Yu, Q.; Zhao, L.P.; Sun, P.; Wang, T.S.; Liu, F.M.; Yan, X.; Gao, Y.; Liang, X.S.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B Chem. 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, Q.; Zhang, S. Simultaneous removal of NOX and SO2 with H2O2 over Fe based catalysts at low temperature. RSC Adv. 2014, 4, 5394–5398. [Google Scholar] [CrossRef]
- Li, Z.; Yi, J.X. Synthesis and enhanced NO2-sensing properties of ZnO-decorated SnO2 microspheres. Mater. Lett. 2019, 236, 570–573. [Google Scholar] [CrossRef]
- Musić, S.; Ristić, M. Adsorption of Zinc (II) on hydrous iron oxides. J. Radioanal. Nucl. Chem. 1992, 162, 351–362. [Google Scholar] [CrossRef]
- Krehula, S.; Musić, S.; Skoko, Ž.; Popović, S. The influence of Zn-dopant on the precipitation of α-FeOOH in highly alkaline media. J. Alloy Comp. 2006, 420, 260–268. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Han, D.; Gu, F. Structural and electronic engineering of 3DOM WO3 by alkali metal doping for improved NO2 sensing performance. Nanoscale 2016, 8, 10622–10631. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Guo, S.; Chen, C.; Sun, L.; Wen, S.; Chen, Y.; Ruan, S. Synthesis of Ni-doped α-MoO3 nanolamella and their improved gas sensing properties. Sens. Actuators B Chem. 2017, 252, 757–763. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.N.; Umar, A.; Chen, W.G.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Li, G.; Han, C.; Wei, D.; Zhong, X.; Zhang, Y.; Ao, Y. Influence of synthesis conditions on microstructure and NO2 sensing properties of WO3 porous films synthesized by non-hydrolytic sol–gel method. Nanomaterials 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, Y.; Zhou, P.; Zhang, J.; Zhang, W.; Chen, X.; Wei, D.; Fang, P.; Shen, Y. Highly selective NO2 sensor based on p-type nanocrystalline NiO thin films prepared by sol–gel dip coating. Ceram. Int. 2018, 44, 753–759. [Google Scholar] [CrossRef]









| Sensing Materials | C(H2S) | Top | R | Tres/Trec | DL (ppm) | Ref |
|---|---|---|---|---|---|---|
| α-Fe2O3 nanospheres | 10 | 228 | 6 | 52 s/- | 1 ppm | [27] |
| α-Fe2O3 nanoparticles | 10 | 300 | 5.5 | 30/5 s | 0.05 | [28] |
| α-Fe2O3 micro-ellipsoids | 10 | 350 | 3 | 80/7 s (0.5 ppm) | 0.5 | [29] |
| Pt:α-Fe2O3 | 10 | 160 | 147.5 | -/- | -/- | [30] |
| Pd:α-Fe2O3 | 10 | 160 | 46.6 | -/- | 10 | [31] |
| Ag:α-Fe2O3 | 100 | 160 | 220 | 42/26 s | 60 | [32] |
| Au:α-Fe2O3 | 10 | 250 | 6.4 | 1.65/27 min | 1 | [33] |
| α-Fe2O3 nanowires | 5 | 175 | 23.5 | 16/174 s | 0.05 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, K.; Zhao, S.; Zhang, W.; Zhong, X.; Li, T.; Cui, B.; Gao, S.; Wei, D.; Shen, Y. Controllable Synthesis of Zn-Doped α-Fe2O3 Nanowires for H2S Sensing. Nanomaterials 2019, 9, 994. https://doi.org/10.3390/nano9070994
Wei K, Zhao S, Zhang W, Zhong X, Li T, Cui B, Gao S, Wei D, Shen Y. Controllable Synthesis of Zn-Doped α-Fe2O3 Nanowires for H2S Sensing. Nanomaterials. 2019; 9(7):994. https://doi.org/10.3390/nano9070994
Chicago/Turabian StyleWei, Kefeng, Sikai Zhao, Wei Zhang, Xiangxi Zhong, Tingting Li, Baoyu Cui, Shuling Gao, Dezhou Wei, and Yanbai Shen. 2019. "Controllable Synthesis of Zn-Doped α-Fe2O3 Nanowires for H2S Sensing" Nanomaterials 9, no. 7: 994. https://doi.org/10.3390/nano9070994







