Fe3O4 Hollow Nanosphere-Coated Spherical-Graphite Composites: A High-Rate Capacity and Ultra-Long Cycle Life Anode Material for Lithium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
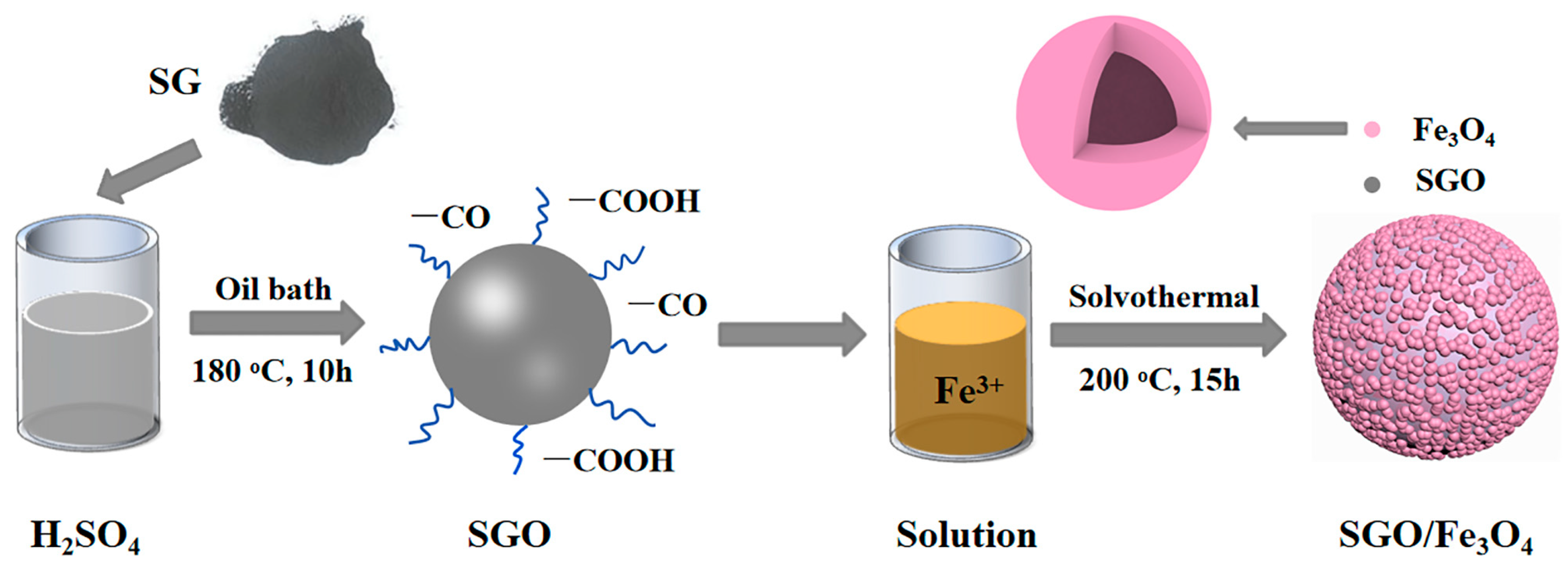
2.1. Oxidation Treatment of SG
2.2. Synthesis of SGO/Fe3O4 Composites
2.3. Material Characterization
2.4. Electrochemical Measurement
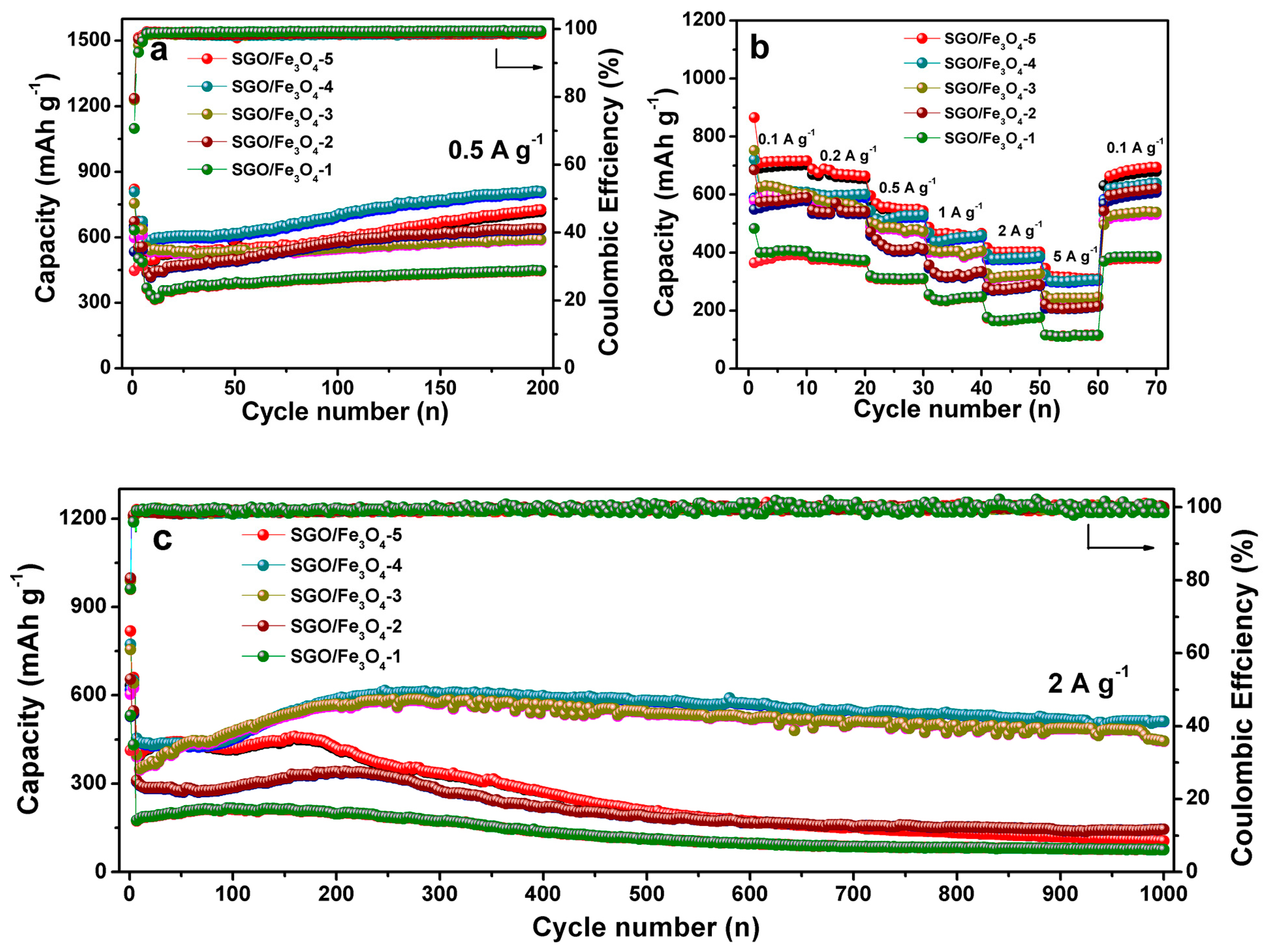
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
- Wakihara, W. Recent developments in lithium ion batteries. Mater. Sci. Eng. 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Wan, C.; Li, H.; Wu, M.; Zhao, C. Spherical natural graphite coated by a thick layer of carbonaceous mesophase for use as anode material in lithium ion batteries. J. Appl. Electrochem. 2009, 39, 1081–1086. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, Y.; Wang, Q.; Zhou, Y. Hierarchical Fe3O4@NC composites: Ultra-long cycle life anode materials for lithium ion batteries. J. Mater. Sci. 2018, 53, 2127–2136. [Google Scholar] [CrossRef]
- Zou, L.; Kang, F.; Zheng, Y.; Shen, W. Modified natural flake graphite with high cycle performance as anode material in lithium ion batteries. Electrochim. Acta 2009, 54, 3930–3934. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Su, L.; Huang, S.; Fang, C.; Wu, Y.; Chou, J.; Wu, N. A proof-of-concept graphite anode with a lithium dendrite suppressing polymer coating. J. Power Sources 2018, 406, 63–69. [Google Scholar] [CrossRef]
- Ong, T.S.; Yang, H. Effect of atmosphere on the mechanical milling of natural graphite. Carbon 2000, 38, 2077–2085. [Google Scholar] [CrossRef]
- Disma, F.; Aymard, L.; Dupond, L.; Tarascon, J.M. Effect of mechanical grinding on the lithium intercalation process in graphites and soft carbons. J. Electrochem. Soc. 1996, 143, 3959–3972. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Luo, G.; Zheng, C.; Xie, D. A rapid and efficient method to prepare exfoliated graphite by microwave irradiation. Carbon 2009, 47, 337–339. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Holze, R. Anode materials for lithium ion batteries by oxidative treatment of common natural graphite. Solid State Ion. 2003, 156, 283–290. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Lee, Y. Performance enhancement of spherical natural graphite by phenol resin in lithium ion batteries. J. Alloys Compd. 2006, 426, 218–222. [Google Scholar] [CrossRef]
- Han, Y.; Kim, J.; An, J.; Hong, I.; Nakabayashi, K.; Miyawaki, J.; Jung, J.D.; Yoon, S.H. Coating of graphite anode with coal tar pitch as an effective precursor for enhancing the rate performance in Li-ion batteries: Effects of composition and softening points of coal tar pitch. Carbon 2015, 94, 432–438. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Tang, Y.; Qi, C.; Ning, G.; Gao, J.; Li, Y. One-step doping-intercalation derived CoO@S-Graphite compound as high performance anode for lithium ion batteries. Energy Technol. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Hu, R.; Ouyang, L.; Zhu, M. Facile synthesis of Fe2O3-graphite composite with stable electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 2017, 125, 421–426. [Google Scholar] [CrossRef]
- Menaehem, C.; Wang, Y.; Flowers, J.; Peled, E.; Greenbaum, S.G. Characterization of lithiated natural graphite before and after mild oxidation. J. Power Sources 1998, 76, 180–185. [Google Scholar] [CrossRef]
- Kumar, Y.P.; Stephan, A.M.; Thayananth, P.; Subramanian, V.; Gopukumar, S.; Renganathan, N.G.; Raghavan, M.; Muniyandi, N. Thermally oxidized graphites as anodes for lithium-ion cells. J. Power Sources 2001, 97, 118–121. [Google Scholar] [CrossRef]
- Taberna, P.L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J.M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, X.; Hu, J.; Guo, Y.; Wan, L. Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv. Funct. Mater. 2010, 18, 3941–3946. [Google Scholar] [CrossRef]
- Baitinger, E.M.; Vekesser, N.A.; Kovalev, I.N.; Ryabkov, L.; Viktorov, V.V. Defect of structure multiwalled carbon nanotubes studied by Raman spectroscopy. Inorg. Mater. 2011, 47, 471–474. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Holze, R. Anode materials for lithium ion batteries from mild oxidation of natural graphite. J. Appl. Electrochem. 2002, 32, 1011–1017. [Google Scholar] [CrossRef]
- Suzuki, S.; Mazej, Z.; Zemva, B.; Ohzawa, Y.; Nakajima, T. Surface passivation of natural graphite electrode for lithium ion battery by chlorine gas. Acta Chim. Slov. 2013, 60, 513–520. [Google Scholar] [PubMed]
- Jin, J.; Deng, H.; Long, D.; Liu, X.; Zhan, L.; Liang, X.; Qiao, W.; Ling, L. Facile synthesis of hierarchically structured Fe3O4/carbon micro-flowers and their application to lithium-ion battery anodes. J. Power Sources 2011, 196, 3887–3893. [Google Scholar] [CrossRef]
- Wen, X.; Wei, X.; Yang, L.; Shen, P. Self-assembled FeS2 cubes anchored on reduced graphene oxide as an anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2090–2096. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Lu, M.; Wen, B.; Ouyang, Q.; Chen, Y.; Zhu, C.; Gao, P.; Li, C.; Cao, M.; et al. Graphene-Fe3O4 nanohybrids: Synthesis and excellent electromagnetic absorption properties. J. Appl. Phys. 2013, 113, 666. [Google Scholar] [CrossRef]
- Xiong, Q.; Tu, J.; Shi, S.; Liu, X.; Wang, X.; Gu, C. Ascorbic acid-assisted synthesis of cobalt ferrite (CoFe2O4) hierarchical flower-like microspheres with enhanced lithium storage properties. J. Power Sources 2014, 256, 153–159. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Zhao, N.; Shi, C.; Liu, E.; Li, J. Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 2013, 7, 4459–4469. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Chen, P.; Zhang, D.; Chen, C. Electrospinning synthesis of C/Fe3O4 composite nanofibers and their application for high performance lithium-ion batteries. J. Power Sources 2008, 183, 717–723. [Google Scholar] [CrossRef]
- Jin, B.; Liu, A.; Liu, G.; Yang, Z.; Zhong, X.; Ma, X.; Yang, M.; Yang, H. Fe3O4-pyrolytic graphite oxide composite as an anode material for lithium secondary batteries. Electrochim. Acta 2013, 90, 426–432. [Google Scholar] [CrossRef]
- Wang, T.; Chen, J.; Zhu, T.; Madhavi, S.; Lou, X. One-pot synthesis of uniform carbon-coated MoO2 nanospheres for high-rate reversible lithium storage. Chem. Commun. 2010, 46, 6906–6908. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.H.; Deshpande, R.; Parilla, P.A.; Whitney, E.; Gillaspie, D.T.; Jones, K.M.; Mahan, A.H.; Zhang, S.; Dillon, A.C. Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv. Mater. 2010, 20, 3627–3632. [Google Scholar] [CrossRef]
- Mao, Y.; Kong, Q.; Guo, B.; Fang, X.; Guo, X.; Shen, L.; Armand, M.; Wang, Z.; Chen, L. Polypyrrole-iron-oxygen coordination complex as high performance lithium storage material. Energy Environ. Sci. 2011, 4, 3442–3447. [Google Scholar] [CrossRef]
- Dang, R.; Jia, X.; Liu, X.; Ma, H.; Gao, H.; Wang, G. Controlled synthesis of hierarchical Cu nanosheets@CuO nanorods as high-performance anode for lithium-ion batteries. Nano Energy 2017, 33, 427–435. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrid with ultrahigh volumetric capacity as anode material for lithium-ion battery. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Guo, L.; Ding, Y.; Qin, C.; Song, W.; Sun, S.; Fang, K.; Li, W.; Du, J.; Wang, F. Anchoring Mn3O4 nanoparticles onto nitrogen-doped porous carbon. J. Alloys Compd. 2018, 735, 209–217. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, Z.; Yang, D.; Sun, T.; Yu, H.; Hoster, H.E.; Hng, H.H.; Zhang, H.; Yan, Q. Hierarchical hollow spheres composed of ultrathin Fe2O3 nanosheets for lithium storage and photocatalytic water oxidation. Energy Environ. Sci. 2013, 6, 987–993. [Google Scholar] [CrossRef]
- Zhao, M.; Torelli, M.; Ren, C.; Ghidiu, C.; Ling, Z.; Anasori, B.; Barsoum, M.; Gogotsi, Y. 2D titanium carbide and transition metal oxides hybrid electrodes for Li-ion storage. Nano Energy 2016, 30, 603–606. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Sun, X.; Li, X.; Wang, D.; Wang, P.; He, D. Three-dimensional network structured a-Fe2O3 made from a stainless steel plate as a high-performance electrode for lithium ion batteries. J. Mater. Chem. A 2013, 21, 6400–6406. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Shan, W.; Xia, X.; Xing, L.; Xue, X. CuO nanorods/graphene nanocomposites for high-performance lithium-ion battery anodes. J. Alloys Compd. 2014, 590, 424–427. [Google Scholar] [CrossRef]
- Sun, X.; Yan, C.; Chen, Y.; Si, W.; Deng, J.; Qswald, S.; Liu, L.; Schmidt, O.G. Three-dimensionally “curved” NiO nanomembranes as ultrahigh rate capability anodes for Li-ion batteries with long cycle life times. Adv. Energy Mater. 2014, 4, 1300912. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Holze, R. Modified natural graphite as anode material for lithium ion batteries. J. Power Sources 2002, 111, 329–334. [Google Scholar] [CrossRef]
- Vargas, O.; Caballero, A.; Morales, J.; Castellon, E.R. Contribution to the understanding of capacity fading in graphene nanosheets acting as an anode in full Li-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Lin, H.; Tu, W.; Chen, X.; Cai, X.; Zheng, X.; Xu, M.; Li, W. A novel fabrication for manganese monoxide/reduced graphene oxide nanocomposite as high performance anode of lithium ion battery. Electrochim. Acta 2016, 198, 66–76. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Yan, X.; Du, R.; Kang, L.; Du, W.; Sun, J.; Zhou, Y. Fe3O4 Hollow Nanosphere-Coated Spherical-Graphite Composites: A High-Rate Capacity and Ultra-Long Cycle Life Anode Material for Lithium Ion Batteries. Nanomaterials 2019, 9, 996. https://doi.org/10.3390/nano9070996
Jiang F, Yan X, Du R, Kang L, Du W, Sun J, Zhou Y. Fe3O4 Hollow Nanosphere-Coated Spherical-Graphite Composites: A High-Rate Capacity and Ultra-Long Cycle Life Anode Material for Lithium Ion Batteries. Nanomaterials. 2019; 9(7):996. https://doi.org/10.3390/nano9070996
Chicago/Turabian StyleJiang, Fuyi, Xinsheng Yan, Rong Du, Litao Kang, Wei Du, Jianchao Sun, and Yanli Zhou. 2019. "Fe3O4 Hollow Nanosphere-Coated Spherical-Graphite Composites: A High-Rate Capacity and Ultra-Long Cycle Life Anode Material for Lithium Ion Batteries" Nanomaterials 9, no. 7: 996. https://doi.org/10.3390/nano9070996
APA StyleJiang, F., Yan, X., Du, R., Kang, L., Du, W., Sun, J., & Zhou, Y. (2019). Fe3O4 Hollow Nanosphere-Coated Spherical-Graphite Composites: A High-Rate Capacity and Ultra-Long Cycle Life Anode Material for Lithium Ion Batteries. Nanomaterials, 9(7), 996. https://doi.org/10.3390/nano9070996




