The Synthesis, Structure, Morphology Characterizations and Evolution Mechanisms of Nanosized Titanium Carbides and Their Further Applications
Abstract
:1. Introduction
2. The Crystal Structure and the Physical and Chemical Properties of Titanium Carbides
2.1. The Crystal Structure of Titanium Carbides
2.2. The Characteristics of the Chemical Bonds in TiCx
2.3. The Relationship between the Crystal Structures and the Growth Morphologies of Titanium Carbides
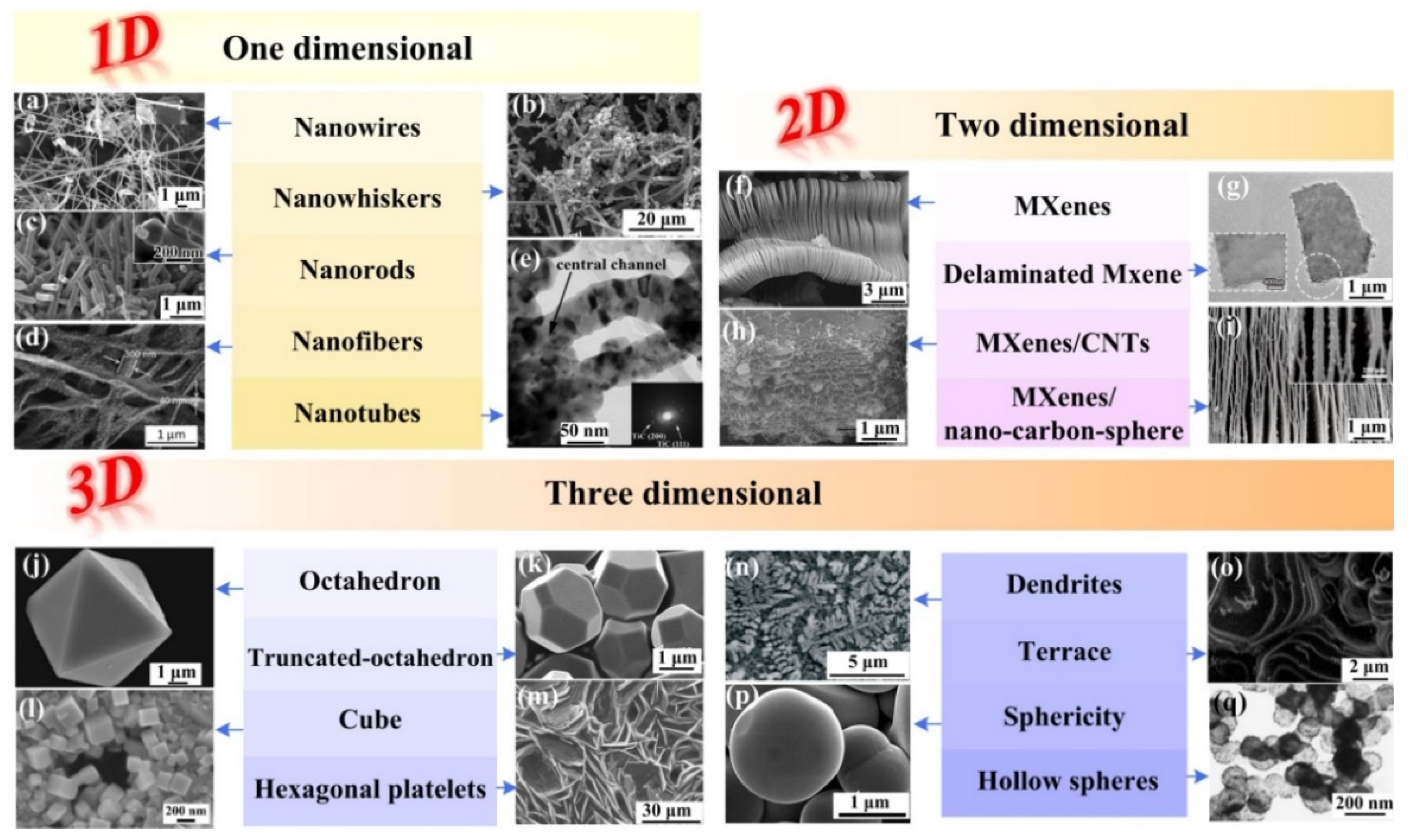
3. The Synthesis and Characterization of One-Dimensional Titanium Carbides as Well as Their Applications
3.1. The Growth of Titanium Carbides during the Chemical Synthesis
3.2. The Electrospinning Technique to Synthesize TiC/C Hybrid Nanomaterials
4. The Synthesis and Characterization of Two-Dimensional Titanium Carbides, as Well as Their Applications
4.1. The Fabrication of Layered Precursor MAX Phases
4.2. The Synthesis Processes of “MXenes” from MAX phases
4.3. More Promising Function Applications of 2D MXences
5. The Synthesis and Characterization of Three-Dimensional Titanium Carbides as Well as Their Applications
5.1. The Synthesis of Three-Dimensional Titanium Carbides in Me-Ti-C Systems (‘Me’ Represents Alloying Elements Al, Cu, Fe, Si and Ni)
5.1.1. The Growth Behaviors of Titanium Carbides in the Al-Ti-C System
- (a)
- Titanium aluminide formation: 2Ti+ 2C + xA1 → TiA1x + Ti + 2C + Q1
- (b)
- Titanium carbide formation: TiAlx + Ti + 2C → TiAlx + TiC + C + Q2
- (c)
- Titanium aluminide decomposition: TiAlx + TiC + C → TiC + Ti + xA1 + C − Q3
- (d)
- Titanium carbide formation: TiC + Ti + xA1 → C + 2TiC + xA1 + Q4
5.1.2. Other Influencing Factors that Change the Morphology of Titanium Carbide in the Al-Ti-C System
5.1.3. Reactions in the Cu-Ti-C System to Synthesize Titanium Carbides with Different Sizes and Morphologies
5.1.4. The Reactions in Fe-Ti-C, Si-Ti-C and Ni-Ti-C Systems to Synthesize Titanium Carbides
5.2. Other Chemical Reaction Methods to Synthesize Titanium Carbides Particles
6. Overview and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, Y.Y.; Qiu, F.; Liu, T.S.; Chu, J.G.; Zhao, Q.L.; Jiang, Q.C. Effects of carbon source on TiC particles’ distribution, tensile, and abrasive wear properties of in situ TiC/Al-Cu nanocomposites prepared in the Al-Ti-C system. Nanomaterials 2018, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Gao, T.; Wu, Y.Y.; Zhang, H.N.; Nie, J.J.; Liu, X.F. Microstructure and mechanical properties at both room and high temperature of in-situ TiC reinforced Al-4.5Cu matrix nanocomposite. J. Alloys Compd. 2018, 767, 606–616. [Google Scholar] [CrossRef]
- Dai, B.Z.; Zhao, B.; Xie, X.; Su, T.T.; Fan, B.B.; Zhang, R.; Yang, R. Novel two-dimensional Ti3C2Tx MXenes/nano-carbon sphere hybrids for high-performance microwave absorption. J. Mater. Chem. C 2018, 6, 5690–5697. [Google Scholar] [CrossRef]
- Liu, Y.; Jian, X.Y.; Su, X.L.; Luo, F.; Xu, J.; Wang, J.B. Electromagnetic interference shielding and absorption properties of Ti3SiC2/nano Cu/epoxy resin coating. J. Alloys Compd. 2018, 740, 68–76. [Google Scholar] [CrossRef]
- Fan, D.; Lu, S.H.; Guo, Y.D.; Hu, X.J. Two-dimensional tetragonal titanium carbide: A high-capacity and high-rate battery material. J. Phys. Chem. C 2018, 122, 15118–15124. [Google Scholar] [CrossRef]
- Chu, X.L.; Fu, Z.M.; Li, S.S.; Zhang, X.L.; Yang, Z.X. Effects of a TiC substrate on the catalytic activity of Pt for NO reduction. Phys. Chem. Chem. Phys. 2016, 18, 13304–13309. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Cheng, L.F.; Zhang, L.T. Synthesis of TiC nanowires on porous ZrSiO4 substrate and their field emission properties. Vacuum 2014, 99, 294–297. [Google Scholar] [CrossRef]
- Tao, X.Y.; Du, J.; Yang, Y.C.; Li, Y.P.; Xia, Y.; Gan, Y.P. TiC nanorods derived from cotton fibers: Chloride-assisted VLS growth, structure, and mechanical properties. Cryst. Growth Des. 2011, 11, 4422–4426. [Google Scholar] [CrossRef]
- Yi, Q.H.; Dai, X.; Zhao, J.; Sun, Y.H.; Lou, Y.H.; Su, X.D.; Li, Q.W.; Sun, B.Q.; Zheng, H.H.; Shen, M.G.; et al. Enhanced mechanical strength and electrical conductivity of carbon-nanotube/TiC hybrid fibers. Nanoscale 2013, 5, 6923–6927. [Google Scholar] [CrossRef]
- Xiong, H.W.; Guo, Y.; Wen, Y.; Lv, Y.P.; Li, Z.Y.; Zhou, K.C. Large-scale synthesis of TiC whiskers by carbothermal reduction with microcrystalline cellulose as the carbon source. J. Crys. Growth 2015, 431, 64–71. [Google Scholar] [CrossRef]
- Taguchi, T.; Yamamoto, H.; Shamoto, S. Synthesis and Characterization of Single-Phase TiC Nanotubes, TiC Nanowires, and Carbon Nanotubes Equipped with TiC Nanoparticles. J. Phys. Chem. C 2007, 111, 18888–18891. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Zou, B.L.; Jiang, Q.C. Morphology evolution of TiCx grains during SHS in an Al-Ti-C. System. Crys. Growth Des. 2009, 9, 646–649. [Google Scholar] [CrossRef]
- Song, M.S.; Huang, B.; Huo, Y.Q.; Zhang, S.G.; Zhang, M.X.; Hu, Q.D.; Li, J.G. Growth of TiC octahedron obtained by self-propagating reaction. J. Cryst. Growth 2009, 311, 378–382. [Google Scholar] [CrossRef]
- Nie, J.F.; Wu, Y.Y.; Li, P.T.; Li, H.; Liu, X.F. Morphological evolution of TiC from octahedron to cube induced by elemental nickel. CrystEngComm 2012, 14, 2213–2221. [Google Scholar] [CrossRef]
- Li, S.B.; Xiang, W.H.; Zhai, H.X.; Zhou, Y. Formation of TiC hexagonal platelets and their growth mechanism. Powder Technol. 2008, 185, 49–53. [Google Scholar] [CrossRef]
- Ma, C.L.; Gu, D.D.; Dai, D.H.; Yu, G.Q.; Xia, M.J.; Chen, H.Y. Thermodynamic behaviour and formation mechanism of novel titanium carbide dendritic crystals within a molten pool of selective laser melting TiC/Ti–Ni composites. CrystEngComm 2017, 19, 1089–1099. [Google Scholar] [CrossRef]
- Zhang, M.X.; Hu, Q.D.; Huang, B.; Li, J.Z.; Li, J.G. Study of formation behavior of TiC in the Fe–Ti–C system during combustion synthesis. Int. J. Refract. Met. Hard Mater. 2011, 29, 356–360. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, F.; Gao, Y.Y.; Dong, B.X.; Shu, S.L.; Lv, M.M.; Yang, H.Y.; Zhao, Q.L.; Jiang, Q.C. Microstructure refinement and strengthening mechanisms of bimodal-sized and dual-phased (TiCn-Al3Tim)/Al hybrid composites assisted ultrasonic vibration. J. Alloys Compd. 2019, 788, 1309–1321. [Google Scholar] [CrossRef]
- Fang, J.X.; Ding, B.J.; Gleiter, H. Mesocrystals: Syntheses in metals and applications. Chem. Soc. Rev. 2011, 40, 5347–5360. [Google Scholar] [CrossRef]
- Geng, R.; Qiu, F.; Jiang, Q.C. Reinforcement in Al Matrix composites: A review of strengthening behavior of nano-sized particles. Adv. Eng. Mater. 2018, 20, 1701089. [Google Scholar] [CrossRef]
- Saba, F.; Sajjadi, S.A.; Haddad-Sabzevar, M.; Zhang, F.M. TiC-modified carbon nanotubes, TiC nanotubes and TiC nanorods: Synthesis and characterization. Ceram. Int. 2018, 44, 7949–7954. [Google Scholar] [CrossRef]
- Zhang, L.F.; Hu, J.J.; Voevodin, A.A.; Fong, H. Synthesis of continuous TiC nanofibers and/or nanoribbons through electrospinning followed by carbothermal reduction. Nanoscale 2010, 2, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Cao, M.S.; Shu, J.C.; Cai, Y.Z.; Wang, X.X.; Zhao, Q.L.; Yuan, J. Atomic layer tailoring titanium carbide MXene to tune transport and polarization for utilization of electromagnetic energy beyond solar and chemical energy. ACS Appl. Mater. Interfaces 2019, 11, 12535–12543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Yin, X.W.; Han, M.K.; Song, C.Q.; Xu, H.L.; Hou, Z.X.; Zhang, L.T.; Cheng, L.F. Ti3C2 MXenes modified with in situ grown carbon nanotubes for enhanced electromagnetic wave absorption properties. J. Mater. Chem. C 2017, 5, 4068–4074. [Google Scholar] [CrossRef]
- Zhang, D.D.; Liu, H.L.; Sun, L.P.; Bai, F.; Wang, Y.; Wang, J.G. Shape-controlled TiCx particles fabricated by combustion synthesis in the Cu-Ti-C system. Crystals 2017, 7, 205. [Google Scholar] [CrossRef]
- Gu, Y.L.; Chen, L.Y.; Li, Z.F.; Qian, Y.T.; Zhang, W.Q. A simple protocol for bulk synthesis of TiC hollow spheres from carbon nanotubes. Carbon 2004, 42, 219–238. [Google Scholar] [CrossRef]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Tian, W.S.; Zhao, Q.L.; Geng, R.; Qiu, F.; Jiang, Q.C. Improved creep resistance of Al-Cu alloy matrix composite reinforced with bimodal-sized TiCp. Mater. Sci. Eng. A 2018, 713, 190–194. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Lin, Q.L.; Zhan, L.; Jiang, Q.C. Growth mechanism of TiCx during self-propagating high-temperature synthesis in an Al-Ti-C System. Crys. Growth Des. 2010, 10, 1590–1597. [Google Scholar] [CrossRef]
- Ni, J.J.; Li, J.; Luo, W.; Han, Q.; Yin, Y.B.; Jia, Z.F. Microstructure and properties of in-situ TiC reinforced copper nanocomposites fabricated via long-term ball milling and hot pressing. J. Alloys Compd. 2018, 755, 24–28. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.W.; Cheng, Y.H.; Niu, C.M. Adsorption and deposition of Li2O2 on TiC {111} surface. J. Phys. Chem. Lett. 2014, 5, 3919–3923. [Google Scholar] [CrossRef] [PubMed]
- Frage, N.; Frumin, N.; Levin, L.; Polak, M.; Dariel, M.P. High-temperature phase equilibria in the Al-rich corner of the Al-Ti-C system. Metall. Mater. Trans. A 1998, 29, 2341. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Mao, J.J.; Li, S.S.; Zhang, Y.X.; Chu, X.L.; Yang, Z.X. The stability of TiC surfaces in the environment with various carbon chemical potential and surface defects. Appl. Surf. Sci. 2016, 386, 202–209. [Google Scholar] [CrossRef]
- Hugosson, H.W.; Korzhavyi, P.; Janson, U.; Johanson, B.; Eriksson, O. Phase stabilities and structural relaxations in substoichiometric TiC1-x. Phys. Rev. B Condens. Matter. 2001, 63, 165116. [Google Scholar] [CrossRef]
- Isaev, E.I. Phonon related properties of transition metals, their carbides, and nitrides: A first-principles study. J. Appl. Phys. 2007, 101, 123519. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lu, H.; Yu, C.; Chen, J.M. First-principles calculations of mechanical properties of TiC and TiN. J. Alloys Compd. 2009, 485, 542–547. [Google Scholar] [CrossRef]
- Sevy, A.; Matthew, D.J.; Morse, M.D. Bond dissociation energies of TiC, ZrC, HfC, ThC, NbC, and TaC. J. Chem. Phys. 2018, 149, 044306. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Jung, Y. TiC-and TiN-Supported Single-Atom Catalysts for Dramatic Improvements in CO2 Electrochemical Reduction to CH4. ACS Energy Lett. 2017, 2, 969–975. [Google Scholar] [CrossRef]
- Grove, D.E.; Gupta, U.; Castleman, A.W. Effect of carbon concentration on changing the morphology of titanium carbide nanoparticles from cubic to cuboctahedron. ACS Nano 2010, 4, 49–54. [Google Scholar] [CrossRef]
- Vines, F.; Sousa, C.; Illas, F.; Liu, P.; Rodriguez, J.A. A Systematic Density Functional Study of Molecular Oxygen Adsorption and Dissociation on the (001) Surface of Group IV-VI Transition Metal Carbides. J. Phys. Chem. C 2007, 111, 16982–16989. [Google Scholar] [CrossRef]
- Wulff, G. On the question of speed of growth and dissolution of crystal surfaces. Z. Krist. 1901, 34, 449–530. [Google Scholar]
- Einstein, T.L. Equilibrium Shape of Crystals. Handbook of Crystal Growth, Fundamentals, 2nd ed.; Nishinaga, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1A (Thermodynamics and Kinetics), Chapter 5; pp. 215–264. [Google Scholar]
- Swaminathan, R.; Willard, M.A.; McHenry, M.E. Experimental observations and nucleation and growth theory of polyhedral magnetic ferrite nanoparticles synthesized using an RF plasma torch. Acta Mater. 2006, 54, 807–816. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ma, F.; Xu, K.W. Calculation of the surface energy of FCC metals with modified embedded-atom method. Appl. Surf. Sci. 2004, 229, 34–42. [Google Scholar] [CrossRef]
- Djellouli, B.; Aourag, H. Theoretical Studies of Stoichiometric TiC. Phys. Status Solidi B 2001, 225, 265–270. [Google Scholar] [CrossRef]
- Ilyasov, V.V.; Pham, K.D.; Yalovega, G.E.; Ershov, I.V.; Ilyasov, A.V.; Nguyen, C.V. First principles investigations of the influence of O-adsorption on the structural and electronic properties of TiC (111) surfaces with vacancies. Surf. Sci. 2016, 649, 20–26. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Cheng, L.F.; Kong, L.; Yin, X.W.; Zhang, L.T. Preparation of titanium carbide nanowires for application in electromagnetic wave absorption. J. Alloys Compd. 2014, 596, 132–139. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Patil, J.V.; Mali, S.S.; Kamble, A.S.; Hong, C.K.; Kim, J.H.; Patil, P.S. Electrospinning: A versatile technique for making of 1D growth of nanostructured nanofibers and its applications: An experimental approach. Appl. Surf. Sci. 2017, 423, 641–674. [Google Scholar] [CrossRef]
- Ren, Y.Q.; Dai, J.; Pang, B.; Liu, X.; Yu, J. Synergistic enhancement of electrochemical performance of electrospun TiC/C hybrid nanofibers for supercapacitor application. Electrochim. Acta 2015, 176, 402–409. [Google Scholar] [CrossRef]
- Cho, D.W.; Park, J.H.; Jeong, Y.; Joo, Y.L. Synthesis of titanium carbide–carbon nanofibers via carbothermal reduction of titania with carbon. Ceram. Int. 2015, 41, 10974–10979. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Xiong, T.R.; Jiang, S.H.; Jian, S.J.; Zhou, Z.P.; Hou, H.Q. Flexible titanium carbide-carbon nanofibers with high modulus and high conductivity by electrospinning. Mater. Lett. 2016, 165, 91–94. [Google Scholar] [CrossRef]
- Fan, Y.P.; Yuan, Z.L.; Zou, G.D.; Zhang, Q.R.; Liu, B.Z.; Peng, Q.M. Two-dimensional MXene/A-TiO2 composite with unprecedented catalytic activation for sodium alanate. Catal. Today 2018, 318, 167–174. [Google Scholar] [CrossRef]
- Lin, S.Y.; Zhang, X.T. Two-dimensional titanium carbide electrode with large mass loading for supercapacitor. J. Power Sources 2015, 294, 354–359. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X=F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Shahin, N.; Kazemi, S.H.; Heidarpour, A. Mechanochemical synthesis mechanism of Ti3AlC2 MAX phase from elemental powders of Ti, Al and C. Adv. Powder Technol. 2016, 27, 1775–1780. [Google Scholar] [CrossRef]
- Liu, Z.W.; Han, Q.Y.; Huang, Z.F.; Xing, J.D.; Gao, Y.M. Sonochemical combustion synthesis of purer Ti2AlC from Ti–Al–C system. Chem. Eng. J. 2016, 288, 532–538. [Google Scholar] [CrossRef]
- Yu, W.B.; Chen, D.Q.; Tian, L.; Zhao, H.B.; Wang, X.J. Self-lubricate and anisotropic wear behavior of AZ91D magnesium alloy reinforced with ternary Ti2AlC MAX phases. J. Mater. Sci. Technol. 2019, 275–284. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Dun, Y.L.; Huo, C.X.; Wang, S.; Cui, C. Thermal stability of two-dimensional Ti2C nanosheets. Ceram. Int. 2015, 41, 2631–2635. [Google Scholar] [CrossRef]
- Hu, M.M.; Hu, T.; Li, Z.J.; Yang, Y.; Cheng, R.F.; Yang, J.X.; Cui, C.; Wang, X.H. Surface functional groups and interlayer water determine the electrochemical capacitance of Ti3C2Tx MXene. ACS Nano 2018, 12, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Shen, X.; Gao, Y.R.; Wang, Z.X.; Yu, R.C.; Chen, L.Q. Atomic-scale recognition of surface structure and intercalation mechanism of Ti3C2X. J. Am. Chem. Soc. 2015, 137, 2715–2721. [Google Scholar] [CrossRef]
- Feng, W.L.; Luo, H.; Wang, Y.; Zeng, S.F.; Tan, Y.Q.; Zhang, H.B.; Peng, S.M. Ultrasonic assisted etching and delaminating of Ti3C2 Mxene. Ceram. Int. 2018, 44, 7084–7087. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Y.F.; Xu, W.T.; Huang, D.C.; Wang, Z.G.; Hong, M.C. Fabrication and thermal stability of two-dimensional carbide Ti3C2 nanosheets. Ceram. Int. 2016, 42, 8419–8424. [Google Scholar] [CrossRef]
- Cui, G.Z.; Sun, X.D.; Zhang, G.Y.; Zhang, Z.; Liu, H.; Gu, J.; Gu, G.X. Electromagnetic absorption performance of two-dimensional MXene Ti3C2Tx exfoliated by HCl+LiF etchant with diverse etching times. Mater. Lett. 2019, 252, 8–10. [Google Scholar] [CrossRef]
- Su, X.H.; Zhang, J.; Mu, H.; Zhao, J.G.; Wang, Z.J.; Zhao, Z.H.; Han, C.X.; Ye, Z.M. Effects of etching temperature and ball milling on the preparation and capacitance of Ti3C2 MXene. J. Alloys Compd. 2018, 752, 32–39. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhou, A.G.; Chen, J.F.; Ji, J.; Zhou, W.J.; Wang, L.B.; Hu, Q.K. Preparation of Ti3C2 and Ti2C MXenes by fluoride salts etching and methane adsorptive properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, L.M.; Tang, H.; Du, F.; Guo, Y.H.; Qiu, T.; Yang, J. Synthesis of two-dimensional Ti3C2Tx MXene using HCl+LiF etchant: Enhanced exfoliation and delamination. J. Alloys Compd. 2017, 695, 818–826. [Google Scholar] [CrossRef]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L.X. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.J.; Wang, L.B.; Zhou, A.G.; Wang, B.; Wang, X.L.; Lian, W.W.; Hu, Q.K.; Qin, G.; Liu, X.Q. Synthesis and Electrochemical Properties of Two-Dimensional RGO/Ti3C2Tx. Nanocomposites 2018, 8, 80. [Google Scholar] [CrossRef]
- Sadeghi, E.; Karimzadeh, F.; Abbasi, M.H. Thermodynamic analysis of Ti–Al–C intermetallics formation by mechanical alloying. J. Alloys Compd. 2013, 576, 317–323. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, M.Y.; Wang, L.J.; Bao, X.H.; Lin, Y.; Shen, J.Y. First-principles study on the structural, phonon, and thermodynamic properties of the ternary carbides in Ti–Al–C system. J. Phys. Status Solidi A 2011, 208, 1879–1884. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Luo, Y.M.; Xu, C.H. Phase and morphology evolution of TiC in the Ti-Si-C system. Int. J. Refract. Met. Hard Mater. 2012, 34, 32–35. [Google Scholar] [CrossRef]
- Choi, Y.; Rhee, S.W. Effects of aluminum addition on the combustion reaction of titanium and carbon to form TiC. J. Mater. Sci. 1993, 28, 6669–6675. [Google Scholar] [CrossRef]
- Lee, W.C.; Chung, S.L. Ignition phenomena and reaction mechanisms of the self-propagating high-temperature synthesis reaction in the Ti+C system. J. Mater. Sci. 1995, 30, 1487–1494. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Wang, J.G.; Jiang, Q.C. Lattice parameter and stoichiometry of TiCx produced in alloyed Ti–C systems by self-propagating high-temperature synthesis. J. Am. Ceram. Soc. 2008, 91, 3813–3816. [Google Scholar] [CrossRef]
- Lee, W.C.; Chung, S.L. Ignition phenomena and reaction mechanisms of the self-propagating high-temperature synthesis reaction in the titanium-carbon- aluminum system. J. Am. Ceram. Soc. 1997, 80, 53–61. [Google Scholar] [CrossRef]
- Liu, Z.W.; Rakita, M.; Xu, W.; Wang, X.M.; Han, Q.Y. Ultrasound assisted combustion synthesis of TiC in Al-Ti-C system. Ultrason. Sonochem. 2015, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Huang, B.; Zhang, M.X.; Li, J.G. Study of formation behavior of TiC ceramic obtained by self–propagating high–temperature synthesis from Al–Ti–C elemental powders. Int. J. Refract. Met. Hard Mater. 2009, 27, 584–589. [Google Scholar] [CrossRef]
- Cochepin, B.; Gauthier, V.; Vrelb, D.; Dubois, S. Crystal growth of TiC grains during SHS reactions. J. Cryst. Growth 2007, 304, 481–486. [Google Scholar] [CrossRef]
- Merzhanov, A.G. The chemistry of self-propagating high-temperature synthesis. J. Mater. Chem. 2004, 14, 1779–1786. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Zhou, D.S.; Jiang, Q.C. Self-propagating high-temperature synthesis of nano-TiCx particles with different shapes by using carbon nano-tube as C source. Nanoscale Res. Lett. 2011, 6, 515. [Google Scholar] [CrossRef]
- Jin, S.B.; Shen, P.; Zhou, D.S.; Jiang, Q.C. A common regularity of stoichiometry-induced morphology evolution of transition metal carbides, nitrides, and diborides during self-propagating high-temperature synthesis. Cryst. Growth Des. 2012, 12, 2814–2824. [Google Scholar] [CrossRef]
- Wang, T.; Gao, T.; Nie, J.F.; Li, P.T.; Liu, X.F. Influence of carbon source on the microstructure of Al-Ti-C master alloy and its grain refining efficiency. Mater. Charact. 2013, 83, 13–20. [Google Scholar] [CrossRef]
- Jiang, H.X.; Sunb, Q.; Zhang, L.L.; Zhao, J.Z. Al-Ti-C master alloy with nano-sized TiC particles dispersed in the matrix prepared by using carbon nanotubes as C source. J. Alloys Compd. 2018, 748, 774–782. [Google Scholar] [CrossRef]
- Yang, Y.C.; Ramirez, C.; Wang, X.; Guo, Z.X.; Tokranov, A.; Zhao, R.Q. Impact of carbon nanotube defects on fracture mechanisms in ceramic nanocomposites. Carbon 2017, 115, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.C. Defects in Carbon Nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef]
- So, K.P.; Lee, I.H.; Duong, D.L.; Kim, T.H.; Lim, S.C.; An, K.H.; Lee, Y.H. Improving the wettability of aluminum on carbon nanotubes. Acta Mater. 2011, 59, 3313–3320. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Qiu, F.; Geng, R.; Chu, J.G.; Zhao, Q.L.; Jiang, Q.C. Effects of nanosized TiCp dispersion on the high-temperature tensile strength and ductility of in situ TiCp/Al-Cu-Mg-Si nanocomposites. J. Alloys Compd. 2019, 774, 425–433. [Google Scholar] [CrossRef]
- Tian, W.S.; Zhao, Q.L.; Zhang, Q.Q.; Qiu, F.; Jiang, Q.C. Simultaneously increasing the high-temperature tensile strength and ductility of nano-sized TiCp reinforced Al-Cu matrix composites. Mater. Sci. Eng. A 2018, 717, 105–112. [Google Scholar] [CrossRef]
- Ding, H.M.; Li, H.; Liu, X.F. Different elements-induced destabilisation of TiC and its application on the grain refinement of Mg-Al alloys. J. Alloys Compd. 2009, 485, 285–289. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, T.; Zhang, H.N.; Nie, J.F.; Liu, X.F. Enhanced age-hardening behavior in Al–Cu alloys induced by in-situ synthesized TiC nanoparticles. J. Mater. Sci. Technol. 2019, 35, 374–382. [Google Scholar] [CrossRef]
- Ruberto, C.; Vojvodic, A.; Lundqvist, B.I. Nature of versatile chemisorption on TiC (111) and TiN (111) surfaces. Solid State Commun. 2007, 141, 48–52. [Google Scholar] [CrossRef]
- Zhou, D.; Jin, S.; Li, Y.; Qiu, F.; Deng, F.; Wang, J.; Jiang, Q. Effect of stoichiometry on the surface energies of {100} and {111} and the crystal shape of TiCx and TiNx. CrystEngComm 2013, 15, 643–649. [Google Scholar] [CrossRef]
- Vasanthakumar, K.; Bakshi, S.R. Effect of C/Ti ratio on densification, microstructure and mechanical properties of TiCx prepared by reactive spark plasma sintering. Ceram. Int. 2018, 44, 484–494. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, T.; Wang, H.C.; Nie, J.F.; Liu, X.F. Influence of C/Ti stoichiometry in TiCx on the grain refinement efficiency of Al–Ti–C master Alloys. J. Mater. Sci. Technol. 2017, 33, 616–622. [Google Scholar] [CrossRef]
- Qiu, F.; Gao, Y.Y.; Liu, J.Y.; Shu, S.L.; Zou, Q.; Zhang, T.Z.; Jiang, Q.C. Effect of C/Ti ratio on the compressive properties and wear properties of the 50 vol.-% submicron-sized TiCx/2014Al composites fabricated by combustion synthesis and hot press consolidation. Powder Metall. 2016, 59, 256–261. [Google Scholar] [CrossRef]
- Dong, B.X.; Yang, H.Y.; Qiu, F.; Li, Q.; Shu, S.L.; Zhang, B.Q.; Jiang, Q.C. Design of TiCx nanoparticles and their morphology manipulating mechanisms by stoichiometric ratios: Experiment and first-principle calculation. Mater. Des. 2019, 181, 107951. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhao, F.; Jiang, Q.C.; Wang, Y.; Ma, B.X. Effect of Mg addition on the self-propagating high temperature synthesis reaction in Al-Ti-C system. J. Mater. Sci. 2005, 40, 1255–1257. [Google Scholar] [CrossRef]
- Yu, R.; He, L.L.; Ye, H.Q. Effects of Si and Al on twin boundary energy of TiC. Acta Mater. 2003, 51, 2477–2484. [Google Scholar] [CrossRef]
- Chien, F.R.; Nutt, S.R.; Cummings, D. Defect structures in single crystal TiC. Philos. Mag. A 1993, 68, 325–348. [Google Scholar] [CrossRef]
- Nie, J.F.; Liu, X.F.; Wu, Y.Y. The influences of B dopant on the crystal structure and nucleation ability of TiCx in the Al melt. Mater. Res. Bull. 2013, 48, 1645–1650. [Google Scholar] [CrossRef]
- Nie, J.F.; Zhao, Y.H.; Wang, E.Z.; Liu, X.F. Study on the evolution processes from TiCx to TiB2 induced by B in Al melt. Mater. Charact. 2015, 100, 68–73. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, J.F.; Gao, T.; Wang, T.; Liu, X.F. Influence of nitrogen on the synthesis and nucleation ability of TiCx in Al-Ti-C master Alloys. J. Alloys Compd. 2014, 601, 267–273. [Google Scholar] [CrossRef]
- Heidarpoura, A.; Aghamohammadib, H.; Jamshidia, R.; Ghasemi, S. The shape evolution of TiCx prepared by mechanical alloying of Ti-Al-C system after HF treatment. Ceram. Int. 2019, 45, 4653–4660. [Google Scholar] [CrossRef]
- Ziemnicka-Sylwester, M. The Cu matrix cermets remarkably strengthened by TiB2 “in situ” synthesized via self-propagating high temperature synthesis. Mater. Des. 2014, 53, 758–765. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, Y.P.; Wang, X.Y.; Koizumi, Y.; Kenta, Y.; Chiba, A. In-situ fabrication and characterization of ultrafine structured Cu–TiC composites with high strength and high conductivity by mechanical milling. J. Alloy Compd. 2016, 657, 122–132. [Google Scholar] [CrossRef]
- Qiu, F.; Han, Y.; Cheng, A.; Lu, J.B.; Jiang, Q.C. Effect of Cr content on the compression properties and abrasive wear behavior of the high-volume fraction (TiC–TiB2)/Cu composites. Acta Metall. Sin. 2014, 27, 951–956. [Google Scholar] [CrossRef]
- Sadeghi, N.; Aghajani, H.; Akbarpour, M.R. Microstructure and tribological properties of in-situ TiC-C/Cu nanocomposites synthesized using different carbon sources (graphite, carbon nanotube and graphene) in the Cu-Ti-C system. Ceram. Int. 2018, 44, 22059–22067. [Google Scholar] [CrossRef]
- Liang, Y.H.; Han, Z.W.; Li, X.J.; Zhang, Z.H.; Ren, L.Q. Study on the reaction mechanism of self-propagating high-temperature synthesis of TiC in the Cu-Ti-C system. Mater. Chem. Phys. 2012, 137, 200–206. [Google Scholar] [CrossRef]
- Akhtar, F.; Askari, S.J.; Shah, K.A.; Du, X.L.; Guo, S.J. Microstructure, mechanical properties, electrical conductivity and wear behavior of high volume TiC reinforced Cu-matrix composites. Mater. Char. 2009, 60, 327–336. [Google Scholar] [CrossRef]
- Wang, X.L.; Ding, H.M.; Qi, F.G.; Liu, Q.; Fan, X.L.; Shi, Y. Mechanism of in situ synthesis of TiC in Cu melts and its microstructures. J. Alloys Compd. 2017, 695, 3410–3418. [Google Scholar] [CrossRef]
- Eremina, M.A.; Lomaeva, S.F.; Burnyshev, I.N.; Kalyuzhnyi, D.G. Mechanosynthesis of precursors for TiC–Cu cermets. Russ. Phys. J. 2018, 60, 2155–2163. [Google Scholar] [CrossRef]
- Ding, H.M.; Wang, X.L.; Liu, Q.; Wang, J.F.; Li, C.Y.; Zhang, X.C. The stability and transformation of TiC with different stoichiometries in Cu–Si melts. Mater. Des. 2017, 135, 232–238. [Google Scholar] [CrossRef]
- Qiang, D.S.; Wang, Q.; Xu, X.X.; Wang, Y.; Li, W.B.; Chen, E.; Li, G.W.; Feng, Y.T.; Fan, X.L.; Ding, H.M. Study of the synthesis process of non-stoichiometric TiC in Cu-Ti melts. Results Phys. 2018, 9, 1564–1569. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.; Song, M.H.; Rhee, W.; Ryu, H.J.; Hong, S.H. In-situ synthesis of TiC/Fe alloy composites with high strength and hardness by reactive sintering. J. Mater. Sci. Technol. 2018, 34, 1397–1404. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Cheng, L.F.; Zhang, L.Y. Controlled fabrication of TiC nanocrystal clusters on surface of Ti particles for application in electromagnetic wave absorption. J. Alloys Compd. 2015, 622, 282–287. [Google Scholar] [CrossRef]
- Zhang, H.M.; Gu, D.D.; Xi, L.X.; Zhang, H.; Xia, M.J.; Ma, C.L. Anisotropic corrosion resistance of TiC reinforced Ni-based composites fabricated by selective laser melting. J. Mater. Sci. Technol. 2019, 35, 1128–1136. [Google Scholar] [CrossRef]
- Zhu, G.L.; Wang, W.; Wang, R.; Zhao, C.B.; Pan, W.T.; Huang, H.J.; Du, D.F.; Wang, D.H.; Shu, D.; Dong, A.P.; et al. Formation Mechanism of Spherical TiC in Ni-Ti-C System during Combustion Synthesis. Materials 2017, 10, 1007. [Google Scholar] [CrossRef]
- Liu, Z.D.; Tian, J.; Li, B.; Zhao, L.P. Microstructure and mechanical behaviors of in situ TiC particulates reinforced Ni matrix composites. Mater. Mater. Sci. Eng. A 2010, 527, 3898–3903. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhang, J.; Zhao, R.Y.; Liang, Y.H.; Jiang, Q.C. The lattice parameter and stoichiometry of TiCx produced in the Ti-C and Ni-Ti-C systems by self-propagating high-temperature synthesis. J. Am. Ceram. Soc. 2008, 91, 2736–2739. [Google Scholar] [CrossRef]
- Meng, H.; Song, K.P.; Wang, H.; Jiang, J.J.; Li, D.; Han, Z.; Zhang, Z.D. Dielectric response of carbon coated TiC nanocubes at 2–18 GHz frequencies. J. Alloys Compd. 2011, 509, 490–493. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]















| Morphology | Size | Preparation Method | Carbide Source | Potentional Applications |
|---|---|---|---|---|
| Nanorods | Diameter 80 to 200 nm Length 1 to 3 μm | Biotemplate method | Cotton T-shirt | Composites reinforcements; Nanoelectromechanical systems [8] |
| Nanowires | Diameter approximately 300 nm Length several microns | Infiltrating and chloride-assisted carbothermal reduction | Phenolic resol | Enhance the emission current for field emission applications [7] |
| Diameter 200–400 nm Length dozens micros | Chloride-assisted carbothermal reaction | Sucrose | Electromagnetic wave absorbing [48] | |
| Nanowhiskers | Diameter 300 nm to 2.5 μm | chloride-assisted carbothermal reduction method | Microcrystalline cellulose | Provide a new method and mechanism to synthesize TiC whiskers [10] |
| TiC/C Nanofibers | Diameter approximately 100 nm | Electrospinning | Polyvinylpyrrolidone | Supercapacitor [51] |
| Unit Cell Parameters (Å) | Volume Change | ||
|---|---|---|---|
| Formula | a = b | c | |
| Ti3AlC2 (Exp.) | 3.080 | 18.415 | |
| Ti3AlC2 | 3.058 | 18.554 | – |
| Ti3C2 | 3.048 | 15.006 | −19% |
| Ti3C2(OH)2 | 3.059 | 19.494 | +5% |
| Ti3C2F2 | 3.019 | 21.541 | +16% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, B.-X.; Qiu, F.; Li, Q.; Shu, S.-L.; Yang, H.-Y.; Jiang, Q.-C. The Synthesis, Structure, Morphology Characterizations and Evolution Mechanisms of Nanosized Titanium Carbides and Their Further Applications. Nanomaterials 2019, 9, 1152. https://doi.org/10.3390/nano9081152
Dong B-X, Qiu F, Li Q, Shu S-L, Yang H-Y, Jiang Q-C. The Synthesis, Structure, Morphology Characterizations and Evolution Mechanisms of Nanosized Titanium Carbides and Their Further Applications. Nanomaterials. 2019; 9(8):1152. https://doi.org/10.3390/nano9081152
Chicago/Turabian StyleDong, Bai-Xin, Feng Qiu, Qiang Li, Shi-Li Shu, Hong-Yu Yang, and Qi-Chuan Jiang. 2019. "The Synthesis, Structure, Morphology Characterizations and Evolution Mechanisms of Nanosized Titanium Carbides and Their Further Applications" Nanomaterials 9, no. 8: 1152. https://doi.org/10.3390/nano9081152





